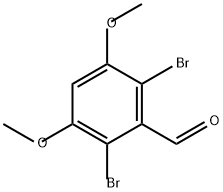
2-Bromo-3,5-dimethoxybenzaldehyde synthesis
- Product Name:2-Bromo-3,5-dimethoxybenzaldehyde
- CAS Number:85565-93-1
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07
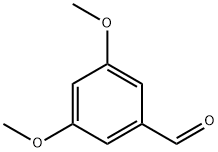
7311-34-4
320 suppliers
$10.00/1g

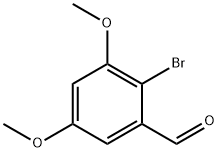
85565-93-1
7 suppliers
inquiry
Yield:85565-93-1 98%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane at 20; for 18 h;
Steps:
2-Bromo-3,5-dimethoxybenzaldehyde (S51)
Small scale: To a solution of 3,5-dimethoxybenzaldehyde (S50, 1.0 equiv, 0.84 g, 5.02 mmol) in DCM (14 mL) at 0 °C was added NBS (1.0 equiv, 0.89 g, 5.02 mmol) portionwise. The reaction was stirred at rt for 18 h. The reaction was then quenched by addition of a saturated solution of NaHCO3 (20 mL) and poured onto water (20 mL). The organics were extracted with DCM, combined, washed with NaHCO3, dried over MgSO4 andconcentrated in vacuo. Pure S51 was obtained as a white solid (1.12 g, 4.55 mmol, 91 %). Gram-scale: Following the same procedure, S51 was obtained as a white solid (14.0 g, 57.1 mmol, 98 %) from3,5-dimethoxybenzaldehyde (S50, 1.0 equiv, 10.0 g, 60.2 mmol) and NBS (1.0 equiv, 10.7 g, 60.2 mmol). Mp: 102-104 °C (lit. 110-112 °C)54; A small difference between the experimental and the reported melting point was noted; 1H NMR (400 MHz, CDCl3) δH 10.40 (s, 1H, CHO), 7.03 (d, J = 2.8 Hz, 1H, ArH), 6.70 (d, J= 2.8 Hz, 1H, ArH), 3.91 (s, 3H, OCH3), 3.84 (s, 3H, OCH3). Spectral data in accordance with those reported in the literature.54
References:
D'Hollander, Agathe C.A.;Westwood, Nicholas J. [Tetrahedron,2018,vol. 74,# 2,p. 224 - 239] Location in patent:supporting information
74726-76-4
2 suppliers
inquiry

85565-93-1
7 suppliers
inquiry
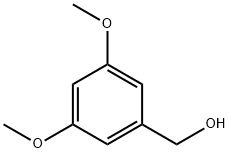
705-76-0
311 suppliers
$10.00/1g

85565-93-1
7 suppliers
inquiry

2150-37-0
292 suppliers
$6.00/5g

85565-93-1
7 suppliers
inquiry