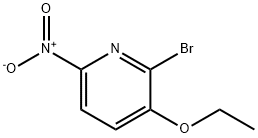
2-Bromo-3-Ethoxy-6-Nitropyridine synthesis
- Product Name:2-Bromo-3-Ethoxy-6-Nitropyridine
- CAS Number:137347-01-4
- Molecular formula:C7H7BrN2O3
- Molecular Weight:247.05

89694-54-2
49 suppliers
inquiry

137347-01-4
8 suppliers
inquiry
Yield:137347-01-4 57%
Reaction Conditions:
with sulfuric acid;nitric acid in water at 0 - 55; for 1 h;
Steps:
C.307 Example 307; N-(5-ethoxypyridin-2-yl)-N'-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]urea.
2-Bromo-3-hydroxy pyridine(11.95 mmol), iodoethane (23.91 mmol), and K2CO3 (21.51 mmol) are diluted in DMF (20 mL) and heated to 80° C. for 2 h. The solvent is removed under reduced pressure and the residue diluted with H2O (20 mL), extracted with EtOAc (3×25 mL). The combined organics are washed with H2O (25 mL), brine (25 mL), dried (MgSO4), and the solvent is removed to give 2-bromo-3-ethoxypyridin as an off-white solid (1.99 g, 83% yield). 2-Bromo-3-ethoxypyridine (0.01 mol) is added to fuming nitric acid (8 mL) and sulfuric acid (8 mL) at 0° C. The clear yellow solution is heated to 55° C. for 1 h, cooled back to RT and added dropwise to ice H2O (400 mL). The solid is filtered to give 2-bromo-3-ethoxy-6-nitropyridine as a light yellow solid (1.40 g, 57% yield). 2-Bromo-3-ethoxy-6-nitropyridine is dissolved in a minimal amount of EtOAc (10 mL) and diluted with EtOH (55 mL). 10% Pd/C catalyst is added as a slurry in EtOAc and the mixture is then put on the Parr apparatus under hydrogen for 1 h (40 psi to 18 psi). The reaction mixture is filtered over Celite to remove the catalyst and the filtrate is concentrated. The residue is diluted with 6M HCl (50 mL), extracted with EtOAc (3×50 mL) and the solvent is removed. The residue is diluted with 1M NaOH (50 mL) and extracted with EtOAc (3×50 mL), dried (MgSO4), and the solvent is removed to give 5-ethoxypyridin-2-amine as a brown oil (722 mg, 94% yield). Example 307 is obtained according to Method C, making non-critical variations. Yield 40%. HRMS (ESI) calculated for C11H10F3N5O2S+H 335.0585 found 335.0584.
References:
US2003/236287,2003,A1 Location in patent:Page 40
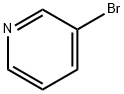
626-55-1
536 suppliers
$5.00/5/ G

137347-01-4
8 suppliers
inquiry
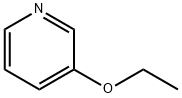
14773-50-3
16 suppliers
inquiry

137347-01-4
8 suppliers
inquiry
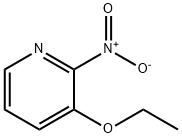
74037-50-6
76 suppliers
$19.00/250mg

137347-01-4
8 suppliers
inquiry