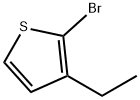
2-Bromo-3-ethylthiophene synthesis
- Product Name:2-Bromo-3-ethylthiophene
- CAS Number:53119-61-2
- Molecular formula:C6H7BrS
- Molecular Weight:191.09
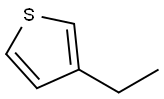
1795-01-3
137 suppliers
$45.00/50mg

53119-61-2
20 suppliers
inquiry
Yield:53119-61-2 100%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 10 - 20; for 4 h;
Steps:
4-Ethyl-5-formylthiophene-2-carboxylic acid 18c
A solution of 3-ethyl-thiophene (12.14g, 54.4 mmol) inacetonitrile (60 mL) is cooled to 10 °C before a solution of N-bromo-succinimide (9.31 g, 52.3 mmol)in acetonitrile (90 mL) is added dropwise. The reaction mixture is stirred andwarmed to rt. Another portion of N-bromo-succinimide(2.0 g, 11.2 mmol) dissolved in acetonitrile (10 mL) is added. After additional1 and 2h, a further portion of N-bromo-succinimide(0.75 g, 4.21 mmol and 0.5 g, 2.81 mmol respectively) is added. Stirring iscontinued for another hour before the solvent is evaporated. The residue isdissolved in DCM, washed twice with 2M aq. NaOH followed by brine, dried overMgSO4, filtered and concentrated. The crude product is purified by distillationat 180 °C/50 mbar to give 2-bromo-3-ethyl-thiophene (11.0 g, quant.) as acolorless liquid; 1H NMR (CDCl3): d 7.22 (d, J = 5.5 Hz,1 H), 6.85 (d, J = 5.5 Hz, 1 H), 2.62 (q, J = 7.5 Hz, 2 H), 1.22(t, J = 7.5 Hz, 3 H).
References:
Lescop, Cyrille;Müller, Claus;Mathys, Boris;Birker, Magdalena;De Kanter, Ruben;Kohl, Christopher;Hess, Patrick;Nayler, Oliver;Rey, Markus;Sieber, Patrick;Steiner, Beat;Weller, Thomas;Bolli, Martin H. [European Journal of Medicinal Chemistry,2016,vol. 116,p. 222 - 238] Location in patent:supporting information