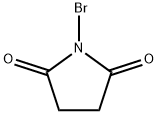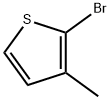
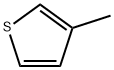
2-Bromo-3-methylthiophene synthesis
- Product Name:2-Bromo-3-methylthiophene
- CAS Number:14282-76-9
- Molecular formula:C5H5BrS
- Molecular Weight:177.06
Yield:-
Reaction Conditions:
with acetic acid in chloroform;water;
Steps:
1 2-Bromo-3-Methylthiophene
Example 1 2-Bromo-3-Methylthiophene To a solution containing 600 ml of chloroform, 600 ml of acetic acid, and 98.0 g of 3-methylthiophene was added portionwise 188.0g of N-Bromosuccinimide with stirring. The addition was exothermic and the reaction mixture was kept below 35°C by means of an ice bath. After the addition was complete, the reaction was allowed to sit for 30 minutes at room temperature whereupon 1.2L of water was added. The organic layer was separated, washed with 600ml of water, followed by 600 ml of 1N NaOH, and finally 600 ml of water. The organic layer was dried over MgSO4, filtered, and the filtrate reduced to a tan oil. Fractional distillation afforded 128g of a yellow mobile oil boiling between 130°C and 170°C.
References:
EP308170,1989,A1
616-44-4
238 suppliers
$18.00/25g

14282-76-9
315 suppliers
$7.00/5g
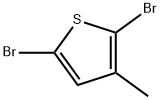
13191-36-1
117 suppliers
$37.07/5g

14282-76-9
315 suppliers
$7.00/5g

616-44-4
238 suppliers
$18.00/25g

14282-76-9
315 suppliers
$7.00/5g

13191-36-1
117 suppliers
$37.07/5g