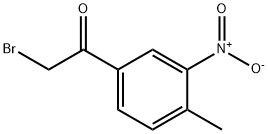
2-bromo-3-nitro-4-methylacetophenone synthesis
- Product Name:2-bromo-3-nitro-4-methylacetophenone
- CAS Number:22019-50-7
- Molecular formula:C9H8BrNO3
- Molecular Weight:258.07

5333-27-7
143 suppliers
$46.10/5g

22019-50-7
36 suppliers
inquiry
Yield:22019-50-7 99%
Reaction Conditions:
with bromine in dichloromethane at 0 - 20;
Steps:
69
Example 692-bromo-1-(4-methyl-3-nitro-phenyl)ethanoneA solution of 1 -(4-methyl-3-nitro-phenyl)ethanone (Sigma-Aldrich, 25.08 g, 0.14 mol) in CH2CI2 (269 mL) is cooled to 0 °C. To this is added bromine (7.17 mL, 0.14 mol) at 0 °C with stirring. The reaction mixture is allowed to warm slowly to rt over a total of 90 minutes. Ice water (500 mL) is added, organics are collected and aqueous layer is extracted with CH2CI2 (2 x 250 mL). The organic layers are combined, washed with water, dried with Na2S04 and concentrated to give the title compound (36.1 g, 99% yield). LCMS m/z = 258.3, 260.2[M+H]+, fR = 2.39 min.
References:
WO2012/88411,2012,A1 Location in patent:Page/Page column 67
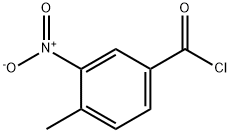
10397-30-5
87 suppliers
$58.20/10ml
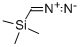
18107-18-1
209 suppliers
$33.00/5g

22019-50-7
36 suppliers
inquiry
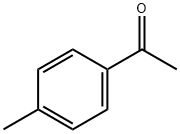
122-00-9
693 suppliers
$5.00/25g

22019-50-7
36 suppliers
inquiry
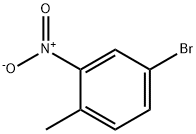
60956-26-5
289 suppliers
$8.00/10g

22019-50-7
36 suppliers
inquiry