
(2-BROMO-4-FLUOROPHENYL)BENZYL ETHER synthesis
- Product Name:(2-BROMO-4-FLUOROPHENYL)BENZYL ETHER
- CAS Number:660842-05-7
- Molecular formula:C13H10BrFO
- Molecular Weight:281.12
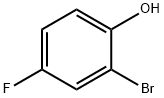
496-69-5
280 suppliers
$6.00/5g
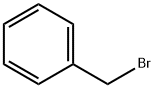
100-39-0
425 suppliers
$10.00/10g

660842-05-7
28 suppliers
$75.00/100mg
Yield:660842-05-7 90%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in DMF (N,N-dimethyl-formamide);aqueous sodium chloride at 20; for 1.5 h;
Steps:
9 Preparation of 2-(4-ethylbenzyl)-4-fluorophenol
Reference Example 9; Preparation of 2-(4-ethylbenzyl)-4-fluorophenol; To a mixture of 2-bromo-4-fluorophenol (24.7 g, 129 mmol), tetrabutylammonium iodide (4.8 g, 13.0 mmol), potassium carbonate (35.9 g, 260 mmol) and N,N-dimethylformamide (390 mL), benzyl bromide (23.5 g, 137 mmol) was added at room temperature and stirred for 1.5 hours. The reaction mixture was poured into a mixture of ethyl acetate and saturated aqueous sodium chloride, and then extracted with ethyl acetate. The organic phase was washed twice with saturated aqueous sodium chloride and then dried over anhydrous magnesium sulfate. After the solvent was distilled off under reduced pressure, the resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate = 90:10-80:20) to give 1-benzyloxy-2-bromo-4-fluoro-benzene (33.0 g, 90%). To a mixture of magnesium (3.2 g, 133 mmol) and tetrahydrofuran (10 mL), 1-benzyloxy-2-bromo-4-fluorobenzene (2-3 mL) was added at room temperature. After heating to start the reaction, a solution of additional 1-benzyloxy-2-bromo-4-fluorobenzene (total 30.0 g, 106 mmol) in tetrahydrofuran (60 mL) was added dropwise and stirred for 30 minutes under the same conditions. After the reaction mixture was cooled in ice, a solution of 4-ethylbenzaldehyde (16.4 g, 77.2 mmol) in tetrahydrofuran (20 mL) was added and stirred at room temperature for 3 hours. The reaction mixture was poured into saturated aqueous ammonium chloride and extracted with ethyl acetate. The organic phase was washed with saturated aqueous sodium chloride and then dried over anhydrous magnesium sulfate. After the solvent was distilled off under reduced pressure, the resulting residue was purified by neutral silica gel column chromatography (hexane:ethyl acetate = 90:10-80:20) to give 2-benzyloxy-5-fluoro-(4'-ethyl)diphenylmethanol. The thus obtained 2-benzyloxy-5-fluoro-(4'-ethyl)-diphenylmethanol, 10% palladium carbon (1.77 g), concentrated hydrochloric acid (3.5 mL) and methanol (350 mL) were mixed and stirred under a hydrogen atmosphere at room temperature for 13 hours. After filtration to remove the insoluble materials, the solvent was distilled off under reduced pressure and the resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate = 90:10-80:20) to give 2-(4-ethylbenzyl)-4-fluorophenol (21.0 g, 85%) as a yellow oil.
References:
EP1528066,2005,A1 Location in patent:Page/Page column 21
452-08-4
258 suppliers
$9.00/1g

660842-05-7
28 suppliers
$75.00/100mg