
2-Bromo-4-(methylsulfonyl)-6-nitrophenol synthesis
- Product Name:2-Bromo-4-(methylsulfonyl)-6-nitrophenol
- CAS Number:20951-41-1
- Molecular formula:C7H6BrNO5S
- Molecular Weight:296.1
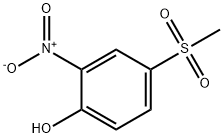
97-10-9
39 suppliers
inquiry

20951-41-1
2 suppliers
inquiry
Yield:20951-41-1 95.9%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 0 - 20; for 2.16667 h;Large scale;
Steps:
1.2a Step 2a. 2-Bromo-4-(methylsulfonyl)-6-nitrophenol (Compound 3)
Step 2a. 2-Bromo-4-(methylsulfonyl)-6-nitrophenol (Compound 3)
N-Bromosuccinimide (NBS, 680 g, 3.82 moles, 1.0 equiv) was added at 0° C. to a solution of 4-(methylsulfonyl)-2-nitro-phenol (Compound 2, 825 g, 3.8 moles) in DMF (5.9 L).
The cooling bath was removed after 10 minutes and the reaction mixture was stirred at room temperature for two hours.
When LCMS indicated the reaction was complete, water (5.9 L) was added and the mixture was stirred at room temperature for one hour.
The solids were filtered, washed with water (3*2.5 L) and dried under vacuum at 45° C. overnight to give 2-bromo-4-(methylsulfonyl)-6-nitrophenol (Compound 3, 1085 g, 1131.1 g theoretical, 95.9% yield) as yellow powder, which was used in the subsequent reaction without further purification.
Compound 3: LCMS calculated for C7H6BrNO5S (M-H)-: 293.9, Found: 294.0; 1H NMR (300 MHz, DMSO-d6) δ 8.33 (d, J=2.0 Hz, 1H), 8.31 (d, J=2.0 Hz, 1H), 3.27 (s, 3H) ppm.
References:
US2017/362229,2017,A1 Location in patent:Paragraph 0260; 0262
14763-60-1
158 suppliers
$11.00/5g

20951-41-1
2 suppliers
inquiry
637-89-8
185 suppliers
$24.00/5g

20951-41-1
2 suppliers
inquiry
1073-72-9
227 suppliers
$6.00/1g

20951-41-1
2 suppliers
inquiry