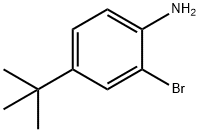
2-Bromo-4-tert-butylaniline synthesis
- Product Name:2-Bromo-4-tert-butylaniline
- CAS Number:103273-01-4
- Molecular formula:C10H14BrN
- Molecular Weight:228.13

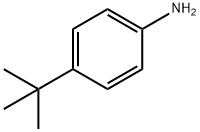
769-92-6
288 suppliers
$6.00/10g

103273-01-4
147 suppliers
$8.00/1g
Yield:103273-01-4 98%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20; for 24 h;
Steps:
7 Synthesis of intermediate 21
35.0 g (201.03 mmol) of NBS was slowly added dropwise to a solution of 30.0 g (201.03 mmol) of 4-tert-butyl aniline in 670 mL of acetonitrile, and the mixture was stirred at room temperature for 24 hours. After completion of the reaction, water was added thereto, followed by extraction with dichloromethane (DCM). The extracted organic layer was washed once with saturated brine and then distilled under reduced pressure. The residue was purified by column chromatography (CHCl3) to obtain 45.0 g (yield: 98.0%) of a yellow liquid compound (Intermediate (21)).
References:
Samsung Display Co., Ltd.;Raepto Co., Ltd.;Cho Hwan-hui;Bae Seong-su;Oh Yu-jin;Kim Gyu-ri;Jeong Hye-in;Han Gap-jong;Kim Nam-ho;Kim Hye-jeong KR2019/25788, 2019, A Location in patent:Paragraph 0157-0160

91801-97-7
6 suppliers
inquiry

103273-01-4
147 suppliers
$8.00/1g

20330-45-4
64 suppliers
$29.00/5g

103273-01-4
147 suppliers
$8.00/1g

769-92-6
288 suppliers
$6.00/10g

103273-01-4
147 suppliers
$8.00/1g
10546-67-5
82 suppliers
$30.00/5g