
2-BROMO-4-TRIFLUOROMETHYLPHENYLISOTHIOCYANATE synthesis
- Product Name:2-BROMO-4-TRIFLUOROMETHYLPHENYLISOTHIOCYANATE
- CAS Number:688763-22-6
- Molecular formula:C8H3BrF3NS
- Molecular Weight:282.08
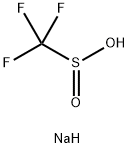
2926-29-6
280 suppliers
$6.00/5g
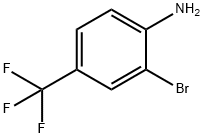
57946-63-1
235 suppliers
$10.00/5g

688763-22-6
14 suppliers
$330.00/5g
Yield:688763-22-6 53.6%
Reaction Conditions:
with copper(l) iodide;phosphonic acid diethyl ester in toluene at 110; for 16 h;Sealed tube;Inert atmosphere;
Steps:
2-bromo-4-(trifluoromethyl)aniline (8 g, 33.33 mmol), CuI(318 mg, 1.67 mmol) and sodium trifluoromethanesulfinate (10.4 g,66.66 mmol) was dissolved in toluene (100 mL) in a sealed bottle.The solution was bubbled with nitrogen and diethyl phosphite(9.206 g, 66.66 mmol)was added. The mixturewas stirred at 110 C.After 16 h, the mixture was poured into H2O and extracted withEtOAc. The organic layer was washed by saturated aqueous NaCland dried over anhydrous Na2SO4. It was filtered and concentratedin vacuo and then purified via flash chromatography on silica gel toget 5.04 g (Yield 53.6%) of 2-bromo-1-isothiocyanato-4-(trifluoromethyl)benzene (8i) as a light green solid. 1H NMR (400 MHz,Chloroform-d) d 7.85 (s, 1H), 7.55 (d, J 8.3 Hz, 1H), 7.35 (d,J 8.3 Hz, 1H).
References:
Cao, Hengyi;Zhu, Guangya;Sun, Lin;Chen, Ge;Ma, Xinxin;Luo, Xiao;Zhu, Jidong [European Journal of Medicinal Chemistry,2019,vol. 183,art. no. 111694]
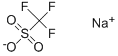
2926-30-9
262 suppliers
$11.00/5g

57946-63-1
235 suppliers
$10.00/5g

688763-22-6
14 suppliers
$330.00/5g
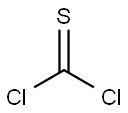
463-71-8
141 suppliers
$32.00/10g

57946-63-1
235 suppliers
$10.00/5g

688763-22-6
14 suppliers
$330.00/5g