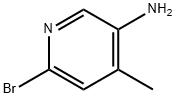
2-BROMO-5-AMINO-4-PICOLINE synthesis
- Product Name:2-BROMO-5-AMINO-4-PICOLINE
- CAS Number:156118-16-0
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
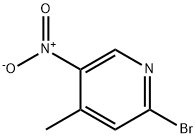
23056-47-5
255 suppliers
$10.00/1g

156118-16-0
145 suppliers
$11.00/250mg
Yield:156118-16-0 97.4%
Reaction Conditions:
with hydrogenchloride;iron;ammonium chloride in ethanol;water at 50 - 80; for 3 h;
Steps:
A suspension of 2-bromo-4-methyl-5-nitropyridine (XIV) (200 g, 921 mmol, 1.00 eq) and NH4C1 (240 g, 4.49 mol, 4.87 eq) in EtOH (3.50 L) and water (150 mL) was heated with stirring to 50°C. To this mixture was added Fe (120 g, 2.15 mol, 2.33 eq) and HC1 (10.2 g, 279 mmol, 0.30 eq). The suspension was then heated to 80°C for another 3 h. The reaction was cooled to 25°C and filtered through a plug of Celite. The filtrate was concentrated under reduced pressure to yield a residue that was taken up in EtOAc (1 Lx 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 6- bromo-4-methylpyridin-3-amine (XV) as brown solid (167.9 g, 898 mmol, 97.4% yield) which was used for the next step without any purification. ‘H NMR (CDC13, 400 MHz) ppm 2.15 (s, 3H), 3.44 (br s, 2H), 7.14 (s, 1H), 7.78 (s, 1H); ESIMS found for C6H7BrN2 mlz 186.8 (M+H).
References:
SAMUMED, LLC.;KC, Sunil Kumar;WALLACE, David Mark;CAO, Jianguo;CHIRUTA, Chandramouli;HOOD, John WO2017/23975, 2017, A1 Location in patent:Paragraph 0610; 0611