2-broMo-6,7,8,9-tetrahydro-5H-carbazole synthesis
- Product Name:2-broMo-6,7,8,9-tetrahydro-5H-carbazole
- CAS Number:78863-99-7
- Molecular formula:C12H12BrN
- Molecular Weight:250.13
Yield: 33%
Reaction Conditions:
with hydrogenchloride in ethanol;water for 1 h;Reflux;
Steps:
21.a
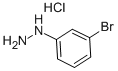
Cyclohexanone (7.7 mL, 73 mmol) was added to a suspension of 3-bromophenyl hydrazine hydrochloride (15 g, 67 mmol) in EtOH (150 mL) and 12 N HCl (30 mL), and the resulting suspension was heated at reflux for 1 h. The suspension was cooled and diluted with H2O (200 mL). The resulting solids were collected by filtration. The solids were dissolved in EtOAc, and the solution was made basic with saturated NaHCO3 solution. The resulting solution was extracted with EtOAc (2×100 mL). The combined organic extracts were dried over Na2SO4, filtered and concentrated to dryness. The solids were suspended in CH2Cl2 (100 mL) and collected by filtration. The solids were washed with MeOH (50 mL) and dried under reduced pressure to provide the title compound (5.74 g, 33%) as a white solid: 1H NMR (500 MHz, CD3OD) δ 7.35 (d, J=1.5 Hz, 1H), 7.22 (d, J=8.0 Hz, 1H), 7.02 (dd, J=8.0, 1.5 Hz, 1H), 2.70 (t, J=6.0 Hz, 2H), 2.64 (t, J=5.5 Hz, 2H), 1.91-1.84 (m, 4H).
References:
ALBANY MOLECULAR RESEARCH, INC. US2011/3793, 2011, A1 Location in patent:Page/Page column 48
591-19-5
333 suppliers
$10.00/10g

78863-99-7
19 suppliers
inquiry

108-94-1
566 suppliers
$12.00/50g
40887-80-7
22 suppliers
inquiry

78863-98-6
6 suppliers
inquiry

78863-99-7
19 suppliers
inquiry