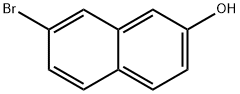
2-Bromo-7-hydroxynaphthalene synthesis
- Product Name:2-Bromo-7-hydroxynaphthalene
- CAS Number:116230-30-9
- Molecular formula:C10H7BrO
- Molecular Weight:223.07

582-17-2
441 suppliers
$24.00/25g

116230-30-9
190 suppliers
$7.00/1g
Yield:116230-30-9 84%
Reaction Conditions:
with bromine;triphenylphosphine in acetonitrile at 0 - 250; for 1.5 h;
Steps:
2.4.1 Synthesis of 7-bromonaphthalen-2-ol (2)
A synthetic procedure was based on the literature method [12]. To a vigorously stirred mixture of triphenylphosphine (31.5g, 120mmol) in acetonitrile (50.0mL), bromine (19.2g, 120mmol) was added dropwise at 0°C. The reaction mixture was allowed to reach room temperature, and 2,7-dihydroxynaphthalene 1 (16.0g, 100mmol) was added in one portion. The mixture was heated to 70°C for 30min, after which the solvent was removed by rotary evaporation. The flask was equipped with a gas trap, and the black residue was heated to 250°C for 1h. After cooling to room temperature, the mixture was dissolved in 200mL of dichloromethane and the viscous liquid was obtained after column chromatography (silica gel, petroleum ether/dichloromethane 1:1). The crude product was purified by column chromatography (silica gel, petroleum ether/dichloromethane 3:2) to give the compound 2 (18.8g, 84.3mmol, 84%) as a beige powder. 1H NMR (400MHz, CDCl3) δ (ppm): 5.09 (s, 1H, -OH), 7.06 (d, 1H, J 2.4Hz, naphthalene-H), 7.10 (dd, 1H, J 8.8, 2.8Hz, naphthalene-H), 7.24 (dd, 1H, J 8.8, 2.0Hz, naphthalene-H), 7.63 (d, 1H, J 8.8Hz, naphthalene-H), 7.72 (d, 1H, J 8.8Hz, naphthalene-H), 7.84 (d, 1H, J 1.2Hz, naphthalene-H). 13C NMR (100MHz, CDCl3) δ (ppm): 108.7, 118.1, 120.8, 127.0, 127.3, 128.3, 129.4, 129.9, 135.7, 154.0. HR-ESI-MS m/z: [M-H]- calcd. for C10H6BrO, 220.9602; found, 220.9601.
References:
Zou, Qi;Li, Xin;Zhou, Ji;Bai, Kangkang;?gren, Hans [Dyes and Pigments,2014,vol. 107,p. 174 - 181]
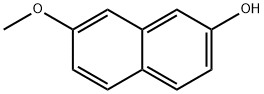
5060-82-2
94 suppliers
$9.00/250mg

116230-30-9
190 suppliers
$7.00/1g
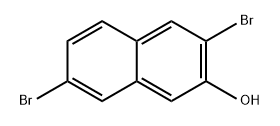
709667-62-9
0 suppliers
inquiry

116230-30-9
190 suppliers
$7.00/1g
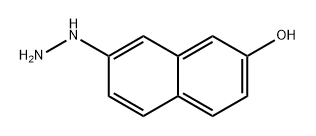
861072-57-3
0 suppliers
inquiry

116230-30-9
190 suppliers
$7.00/1g