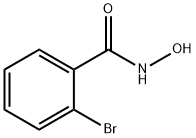
2-bromo-N-hydroxybenzamide synthesis
- Product Name:2-bromo-N-hydroxybenzamide
- CAS Number:2593-27-3
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03

610-94-6
304 suppliers
$13.00/25g

2593-27-3
11 suppliers
inquiry
Yield:2593-27-3 60%
Reaction Conditions:
with hydroxylamine hydrochloride;potassium hydroxide in methanol at 0 - 20; for 20.5 h;
Steps:
Method c
General procedure: Unsubstituted or N-methylated hydroxylamine hydrochloride (13.4 mmol) was dissolved in hot MeOH (5 mL) and KOH (1.12 g, 20 mmol) in MeOH (4 mL) was added and mixture was immediately cooled to 0 °C for 30 min. Methyl ester of acid (6.7 mmol) was added and mixture was incubated at room temperature for 20 hours. Then AcOH (0.6 mL) was added and solvent was removed at reduced pressure. The residue was diluted with water, extracted with ethylacetate, and dried over Na2SO4. The solvent was removed at reduced pressure, and the oil was chromatographed on SiO2, eluting with CHCl3/EtOH, to give the raw product that was finally purified by washing with appropriate organic solvents. Yields 20-60 %.
References:
Kozlov, Maxim V.;Kleymenova, Alla A.;Romanova, Lyudmila I.;Konduktorov, Konstantin A.;Smirnova, Olga A.;Prasolov, Vladimir S.;Kochetkov, Sergey N. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 21,p. 5936 - 5940] Location in patent:supporting information

88-65-3
339 suppliers
$5.00/5g

2593-27-3
11 suppliers
inquiry
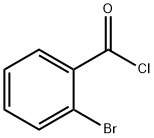
7154-66-7
166 suppliers
$10.00/5g

2593-27-3
11 suppliers
inquiry
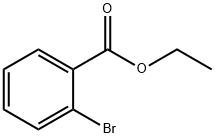
6091-64-1
173 suppliers
$10.00/1g

2593-27-3
11 suppliers
inquiry