
2-BROMO-N-METHOXY-N-METHYLPYRIDINE-4-CARBOXAMIDE synthesis
- Product Name:2-BROMO-N-METHOXY-N-METHYLPYRIDINE-4-CARBOXAMIDE
- CAS Number:656257-69-1
- Molecular formula:C8H9BrN2O2
- Molecular Weight:245.07
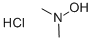
16645-06-0
31 suppliers
$306.00/25g
66572-56-3
341 suppliers
$7.00/5g

656257-69-1
25 suppliers
$51.00/100mg
Yield:656257-69-1 99%
Reaction Conditions:
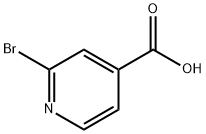
Stage #1: 2-bromoisonicotinic acidwith triethylamine;HATU in N,N-dimethyl-formamide at 20 - 30; for 0.333333 h;
Stage #2: N,N-dimethylhydroxylamine hydrochloride in N,N-dimethyl-formamide at 20 - 30;
Steps:
2.1 The first step 2-bromo-N-methoxy-N-methylisonicotinamide 2b
2-Bromo-4-pyridinecarboxylic acid 2a (4.0g, 19.80mmol), HATU (11.28g, 29.67mmol) and triethylamine (6.0g, 59.29mmol) dissolved in N,N-dimethylformamide (80mL )in,Stir at room temperature for 20 minutes, add dimethylhydroxylamine hydrochloride (2.32 g, 23.78 mmol), react at room temperature overnight, and TLC monitors most of the raw material reaction. The reaction solution was diluted with water, extracted with ethyl acetate, combined the organic phases, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and purified by silica gel column chromatography to obtain the title compound 2b as a pale yellow liquid (4.8g, yield 99%)
References:
CN112812105,2021,A Location in patent:Paragraph 0075; 0127-0132

6638-79-5
559 suppliers
$6.00/25g

66572-56-3
341 suppliers
$7.00/5g

656257-69-1
25 suppliers
$51.00/100mg

66572-56-3
341 suppliers
$7.00/5g

656257-69-1
25 suppliers
$51.00/100mg