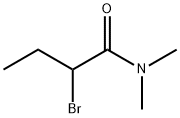
2-bromo-N,N-dimethylbutyramide synthesis
- Product Name:2-bromo-N,N-dimethylbutyramide
- CAS Number:39221-60-8
- Molecular formula:C6H12BrNO
- Molecular Weight:194.07
Yield:39221-60-8 56%
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 20;
Steps:
419.1 STEP 1: (R)-2-BROMO-N.N-DTMETHYLBUTANAMIDE AND (S)-2- BROMO-NN-DIMETHYLBUTANAN’IIDE
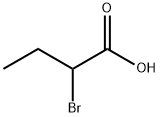
To a iOO-mL rotmd-bottomed flask were added 2-(ihhenzotriazoI-1-yfl-1.1 ,3,3-1etramethyiuronium hexafluorophosphate (5 79 g, 15 3 mmoi), DIEA(356 ml. 20.4 rnrnoi), 2-bromo butyric acid (1.70 g, 10.2 rnrnoi) anddimethylamine, 2.0 M solution in tetrahydrofuran (763 ml, 15.3 rnrnol) in THF(40.7 ml). The mixture was stirred at ambient temperature overnight. The reactionmixture was diluted with waler (30 mL) and extracted with CH2CL2 (2 x 30 inL). The organic solution was concentrated. The crude material was purified by chromatography through a Redi -Sep pre-packed silica gel column (40 g), eluting with a gradient of 0% to 65% EtOAc in heptane, to provide the title compound(110g. 56%). ‘HNMR (400 MHz, CDCIs) ? 431-4.42 (m, 11-1), 3.13 (s, 3Ff),3.03 (s, 3H), 2.00-228 (in. 21-1), 1.04 (t, J=7.24 Hz, 31-1).
References:
WO2017/147410,2017,A1 Location in patent:Page/Page column 1098
26074-52-2
116 suppliers
$65.00/1g

124-40-3
510 suppliers
$18.00/100ml

39221-60-8
9 suppliers
inquiry