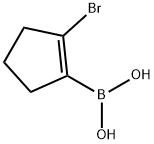
2-broMocyclopent-1-enylboronic acid synthesis
- Product Name:2-broMocyclopent-1-enylboronic acid
- CAS Number:612833-43-9
- Molecular formula:C5H8BBrO2
- Molecular Weight:190.83
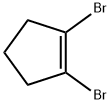
75415-78-0
109 suppliers
$70.00/100mg

612833-43-9
22 suppliers
inquiry
Yield:612833-43-9 26%
Reaction Conditions:
Stage #1: 1,2-dibromocyclopentenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.666667 h;
Stage #2: with Triisopropyl borate in tetrahydrofuran;hexane at 20;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane at 20; for 0.25 h;
Steps:
B.i
General Procedure B General Procedure B (i) 2-bromocyclopent-1-enylboronic acid 1, 2-dibromocyclopentene (10.1 g, 0.044 mol) was dissolved in 100 mL of tetrahydrofuran, cooled to-78°C and n-butyllithium, 1.6 M solution in hexane (28 mL, 0.044 mol), was added dropwise over 20 minutes under nitrogen. The mixture was stirred at-78°C for further 20 minutes, then triisopropylborate (20.8 mL, 0.089 mol) was added dropwise. The cooling bath was then removed and the reaction mixture was allowed to reach room temperature. The reaction mixture was then quenched with 1M HCI (40 mL) and stirred vigorously at room temperature for 15 minutes. The organic layer was then separated, dried over magnesium sulphate and evaporated down. The residue was triturated with dichloromethane to yield the title compound as a white solid (2. 2g, 26%). 'H NMR (CD30D) : 1.92-1. 98 (2H, m), 2.50-2. 55 (2H, m), 2.73-2. 78 (2H, m), 5.02 (2H, s).
References:
WO2003/84917,2003,A1 Location in patent:Page/Page column 63