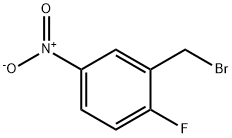
2-BROMOMETHYL-1-FLUORO-4-NITROBENZENE synthesis
- Product Name:2-BROMOMETHYL-1-FLUORO-4-NITROBENZENE
- CAS Number:454-15-9
- Molecular formula:C7H5BrFNO2
- Molecular Weight:234.02
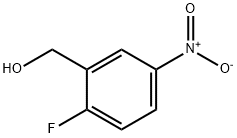
63878-73-9
71 suppliers
$12.00/250mg

454-15-9
57 suppliers
$28.00/250mg
Yield: 98%
Reaction Conditions:
with phosphorus tribromide in chloroform at 37 - 60;
Steps:
9 Preparation of 2-(bromomethyl)-1-fluoro-4-nitrobenzene. Benzyl Alcohol/Phosphorus Tribromide Method
70.0 g (0.41 mol) of 2-fluoro-5-nitrobenzyl alcohol were introduced as initial charge in 470 ml of chloroform, and 55.4 g (0.21 mol) of phosphorus tribromide were added.
During this addition, the temperature increased to 37° C.
The reaction mixture was stirred for min at 60° C.
After cooling to RT, the mixture was neutralized (pH=7) by adding saturated aqueous sodium hydrogen carbonate solution.
The organic phase was separated off and the aqueous phase was extracted with 50 ml of dichloromethane.
The combined organic phases were dried over sodium sulphate, filtered and evaporated to dryness on a rotary evaporator.
The solid obtained was dried in vacuo.
This gave 93.7 g (98%) of the desired compound, which was reacted further without purification.
References:
BAYER INTELLECTUAL PROPERTY GMBH;Krüger, Joachim;Grossbach, Danja;Paulsen, Holger;Kroh, Walter US2013/116443, 2013, A1 Location in patent:Paragraph 0117; 0118
455-88-9
290 suppliers
$6.00/10g

454-15-9
57 suppliers
$28.00/250mg
446-48-0
333 suppliers
$6.00/10g

454-15-9
57 suppliers
$28.00/250mg
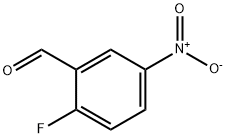
27996-87-8
176 suppliers
$5.00/1g

454-15-9
57 suppliers
$28.00/250mg
![PROPANENTRILE,2,2'-[(1E)-1,2-DIAZENEDIYL]BIS(2-METHYLPROPIONITRILE)](/CAS/20180808/GIF/34241-39-9.gif)