
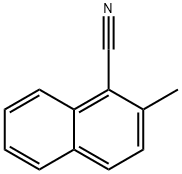
2-(BroMoMethyl)-1-naphthonitrile synthesis
- Product Name:2-(BroMoMethyl)-1-naphthonitrile
- CAS Number:67266-37-9
- Molecular formula:C12H8BrN
- Molecular Weight:246.1
20944-85-8
12 suppliers
inquiry

67266-37-9
6 suppliers
inquiry
Yield:67266-37-9 85%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in tetrachloromethane; for 4 h;Reflux;
Steps:
4.2. Preparation of 1-Cyano-2-[2,2-dicyano-2-(cyclopent-2-en-1-yl)ethyl]naphthalene (1a)
To a stirred CCl4 (20 mL) solution of cyclopentene (2.94 g,43.1 mmol) were added N-bromosuccinimide (8.06 g, 45.3 mmol)and benzoyl peroxide (catalytic amount). The solution was stirredat reflux for 1 h. The formed solid was removed byfiltration. Thefiltrate was concentrated in vacuo to give 3-bromocyclopentene,which was used to further reaction without purification.To a stirred suspension of NaH (60% in mineral oil, 2.60 g,65.0 mmol) in THF (10 mL) was slowly added a THF (5 mL) solutionof malononitrile (5.70 g, 86.2 mmol) at 0 °C under argon atmosphere.The suspension was stirred for 1 h at room temperature. ATHF (5 mL) solution of 3-bromocyclopentene was slowly added at0 °C and the solution was stirred for 2 h at room temperature. Et2Oand brine were added and the organic layer was separated, driedover Na2SO4,filtered, and concentrated in vacuo. The residue wassubjected to silica gel column chromatography (eluent; hexane-AcOEt) to give 3-(dicyanomethyl)cyclopentene (0.889 g,6.73 mmol, 16% yield). To a stirred CCl4 (60 mL) solution of 2-methylnaphthalene(20.1 g, 141 mmol), Fe powder (catalytic amount) and I2 (catalyticamount) were slowly added a CCl4 (10 mL) solution of Br2 (22.5 g,7.2 mL, 141 mmol) at 0 °C. The solution was stirred for 3 h. SatNa2S2O3 aq was added. The organic layer was washed with brine,dried over Na2SO4,filtered, and concentrated in vacuo. The residuewas subjected to silica gel column chromatography (eluent;hexane) to give 1-bromo-2-methylnaphthalene (29.3 g, 133 mmol,94% yield). A mixture of 1-bromo-2-methylnaphthalene (20.2 g,91.3 mmol), CuCN (22.6 g, 252 mmol), N-methylpyrrolidone(50 mL) was stirred at 200 °C for 40 min. After the mixture wascooled to room temperature, 25% NH3 aq (40 mL) and CHCl3 wereadded. The separated organic layer was concentrated in vacuo. The residue was subjected to silica gel column chromatography(eluent; hexane-AcOEt) followed by recrystallization from EtOHto give 1-cyano-2-methylnaphthalene (11.9 g, 71.1 mmol, 79%yield). To a stirred CCl4 solution (50 mL) of 1-cyano-2-methylnaph-thalene (8.10 g, 48.4 mmol) was added N-bromosuccinimide(10.1 g, 56.7 mmol) and benzoyl peroxide (catalytic amount), andthe solution was stirred at reflux for 4 h. The solid was removed byfiltration. Thefiltrate was concentrated in vacuo. The residue wassubjected to silica gel column chromatography (eluent; hexane-AcOEt) followed by recrystallization from EtOH to give 2-bromomethyl-1-cyanonaphthalene (10.1 g, 41.0 mmol, 85% yield). To a stirred suspension of NaH (60% in mineral oil, 88 mg,2.2 mmol) in THF (30 mL) was slowly added THF (10 mL) solution of3-(dicyanomethyl)cyclopentene (250 mg, 1.89 mmol) at 0 °C underargon atmosphere. The solution was stirred at room temperaturefor 1 h. To the solution was added a THF (20 mL) solution of 2-bromomethyl-1-cyanonaphthalene (492 mg, 2.0 mmol) at 0 °C,and the solution was stirred at room temperature for 1 h. Brine andEt2O were added. The organic layer was dried over Na2SO4,filtered,and concentrated in vacuo. The residue was subjected to silica gelcolumn chromatography (eluent; hexane:AcOEt = 5:1) followed byrecycling preparative HPLC (GPC, eluent; CHCl3) to give 1-cyano-2-[2,2-dicyano-2-(cyclopent-2-en-1-yl)ethyl]naphthalene (1a,329 mg, 1.11 mmol, 61% yield). Colorless solid; mp 135.5-137.5 °C; 1H NMR (300 MHz, CDCl3) δ 2.07-2.16 (m, 1H), 2.37-2.56 (m, 2H), 2.65-2.76 (m, 1H), 3.46-3.52 (m, 1H), 3.58 (d,J = 14.1 Hz,1H), 3.72 (d, J = 14.1 Hz,1H), 5.87-5.91 (m,1H), 6.22-6.26(m, 1H), 7.63-7.76 (m, 3H), 7.94 (d, J = 8.1 Hz, 1H), 8.13 (d, J = 8.5 Hz,1H), 8.28 (dd, J = 8.4, 0.5 Hz, 1H) ppm; 13C NMR (75 MHz, CDCl3) δ26.7, 32.7, 40.0, 43.8, 54.1, 111.9, 114.3, 114.5, 116.3, 125.7, 126.1,126.5, 128.1, 128.5, 129.2, 132.5, 132.6, 133.5, 136.5, 138.7 ppm; IR(KBr) ν 2219, 2246 cm-1; MS (EI) m/z 297 (M+).
References:
Maeda, Hajime;Uesugi, Tomoya;Fujimoto, Yasufumi;Mukae, Hirofumi;Mizuno, Kazuhiko [Journal of Photochemistry and Photobiology A: Chemistry,2017,vol. 337,p. 198 - 206]
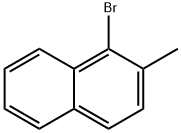
2586-62-1
161 suppliers
$10.00/5g

67266-37-9
6 suppliers
inquiry
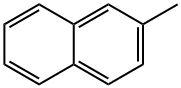
91-57-6
252 suppliers
$13.00/5g

67266-37-9
6 suppliers
inquiry