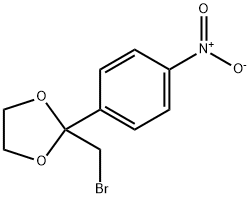
2-(BROMOMETHYL)-2-(4-NITROPHENYL)-1,3-DIOXOLANE synthesis
- Product Name:2-(BROMOMETHYL)-2-(4-NITROPHENYL)-1,3-DIOXOLANE
- CAS Number:3418-28-8
- Molecular formula:C10H10BrNO4
- Molecular Weight:288.09
Yield:3418-28-8 98%
Reaction Conditions:
with N-Bromosuccinimide at 45; for 24 h;
Steps:
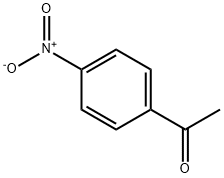
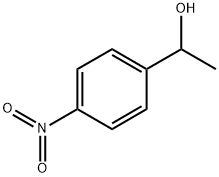
General procedure for preparation of a-haloacetal ofacetophenones from acetophenons
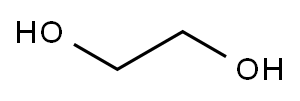
General procedure: A mixture of 2 mmol of substrate, and 6 mL ethylene glycol was stirred for 5 min, and then 4.6 mmol of NBS or 8 mmol of NCS was added by 5 times in one hour, and the mixture was stirred 24 h at room temperature or 45 C. After ward, the mixture was extracted with ether (10 mL3). The combined organic layer was washed twice by water (20 mL), and dried over anhydrous Na2SO4. After filtration, the solvent was removed under reduced pressure, and the residual was treated with alumina chromatography (Petroleumether/AcOEt20/1,v/v) to generate the product.
References:
Zheng, Zubiao;Han, Bingbing;Wu, Fang;Shi, Tengfei;Liu, Jie;Zhang, Yong;Hao, Jialong [Tetrahedron,2016,vol. 72,# 48,p. 7738 - 7743]