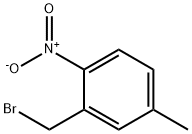
(2-Bromomethyl)-4-methyl-1-nitrobenzene synthesis
- Product Name:(2-Bromomethyl)-4-methyl-1-nitrobenzene
- CAS Number:110822-05-4
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06

66424-92-8
67 suppliers
$26.00/250mg

110822-05-4
27 suppliers
inquiry
Yield:110822-05-4 95%
Reaction Conditions:
with phosphorus tribromide in dichloromethane at 20; for 0.5 h;
Steps:
a. PREPARATION OF 2-(BROMOMETHYL)-4-METHYL-1-NITROBENZENE (COMPOUND 49
a. PREPARATION OF 2-(BROMOMETHYL)-4-METHYL-1-NITROBENZENE (COMPOUND 49)O2NBr&L[00273j 5-methyl-2-nitrobenzoic acid (10.0 g, 55.2 mmol) were dissolved in THF (100 mL) followed by dropping 2.0 M borane dimethlsulfide comples (110 mL, 221.1 mmol) and heating at 80 °C. After 1.5 hours, lOOml of 1 M HC1 were dropped into this reaction system while cooling with ice and stirring. The system was extracted with ethyl acetate, and then was dried with Na2SO4 followed by concentration under reduced pressure and drying to obtain benzylic alcohol (9.1 g, 99% yield) as viscous colorless oil. (5-methyl-2-nitrophenyl) methanol (9.1 g, 54.5 mmol ) were dissolved in dry CH2C12 (100 mL) followed by the addition of phosphorous tribromide (7.4 g, 27.3 mmol) and stirring at room temperature. After 30 minutes, saturated NaHCO3(aq. 100 mL ) were added followed by stirring for 10 minutes and extracting with CH2C12 (200 ml x2). The organic phase was then concentrated under reduced pressure and dried to obtain the benzyl bromide analogue, 49, (11.9 g, 95% yield) as a white solid: mp 51 -52 °C.
References:
WO2013/120045,2013,A1 Location in patent:Paragraph 00273
3113-72-2
293 suppliers
$10.00/5g

110822-05-4
27 suppliers
inquiry