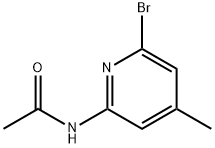
2-Bromoo-4-methyl-6-acetaminopyridine synthesis
- Product Name:2-Bromoo-4-methyl-6-acetaminopyridine
- CAS Number:263894-96-8
- Molecular formula:C8H9BrN2O
- Molecular Weight:229.07

108-24-7
5 suppliers
$14.00/250ML
73895-98-4
78 suppliers
$7.00/250mg

263894-96-8
7 suppliers
inquiry
Yield:263894-96-8 89%
Reaction Conditions:
with pyridine;dmap
Steps:
G.4
Peracetylation of the mixture containing N- (6-bromo-4-methyl-pyridin-2-yl)-acetamide and 2-amino-6- bromo-4-methyl-pyridine (compound H1) can be performed by a person skilled in the art according to Einhorn's procedure, thereby using pyridine, acetic anhydride, and N, N-dimethylaminopyridine. The pure title compound is obtained as a colourless, amorphous solid in up to 89% yield after chromatography on silica gel (eluent : toluene/ethyl acetate 4: 1). M. p. 190°C. TSP-MS: 228.9/230. 8 (M) T ; 92%, 100%). TLC: Rf = 0. 50 (toluene/ethyl acetate 4: 1).
References:
WO2005/61496,2005,A1 Location in patent:Page/Page column 47