
2-Butanone, 1-(4-methoxyphenyl)- synthesis
- Product Name:2-Butanone, 1-(4-methoxyphenyl)-
- CAS Number:53917-01-4
- Molecular formula:C11H14O2
- Molecular Weight:178.23
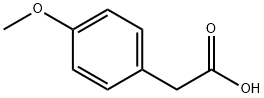
104-01-8
533 suppliers
$6.00/10g
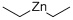
557-20-0
235 suppliers
$14.00/1g

53917-01-4
19 suppliers
$174.16/0.25g
Yield:53917-01-4 99%
Reaction Conditions:
Stage #1: 4-Methoxyphenylacetic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 0 - 20; for 1.5 h;
Stage #2: diethylzincwith tetrakis(triphenylphosphine) palladium(0) in hexane;benzene at 0 - 20; for 15 h;Negishi Coupling;
Steps:
Typical Procedure for theNegishi Coupling Reaction
General procedure: Oxalyl chloride (0.943 mL, 11.0 mmol) was added to a solution of2-(4-fluorophenyl)acetic acid (1.00 g, 6.49 mmol) in CH2Cl2 (15mL) at 0 °C. The reaction was initiated by the addition of 5 drops ofDMF. After 30 min at 0 °C, the mixture was allowed to warm to r.t.and stirred for an additional hour. The solvents were evaporated andthe residue was dissolved in benzene (15 mL). Then, Pd(PPh3)4 (300mg, 0.260 mmol) was added. After cooling to 0 °C, 1.06 M Et2Znin n-hexane (6.12 mL, 6.49 mmol) was added dropwise and the mixturewas stirred at r.t. for 15 h. After quenching by the addition ofwater, the mixture was filtered over a Celite pad and the filtratewas extracted with EtOAc (3 ×). The combined organic layers werewashed with brine, dried (Na2SO4), filtered, and concentrated invacuo. The crude product was purified by column chromatography(silica gel, n-hexane-EtOAc, 10:1) to give 7b (704 mg, 70%) as acolorless liquid
References:
Miura, Takuya;Fujioka, Saki;Takemura, Naoto;Iwasaki, Hiroki;Ozeki, Minoru;Kojima, Naoto;Yamashita, Masayuki [Synthesis,2014,vol. 46,# 4,art. no. SS-2013-F0692-OP,p. 496 - 502]
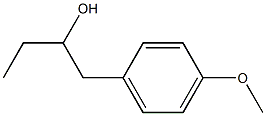
110661-90-0
5 suppliers
inquiry

53917-01-4
19 suppliers
$174.16/0.25g

104-93-8
396 suppliers
$6.00/25g
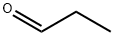
123-38-6
443 suppliers
$14.00/5mL

53917-01-4
19 suppliers
$174.16/0.25g
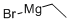
925-90-6
353 suppliers
$12.00/5ml
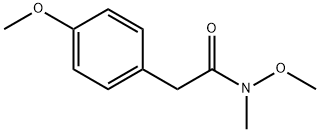
267884-96-8
9 suppliers
$800.00/5G

53917-01-4
19 suppliers
$174.16/0.25g

109368-12-9
2 suppliers
inquiry

53917-01-4
19 suppliers
$174.16/0.25g