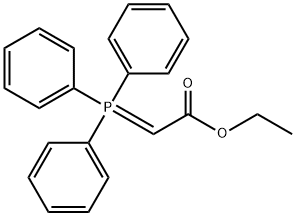
2-Butenoic acid, 4,4-dimethoxy-, ethyl ester synthesis
- Product Name:2-Butenoic acid, 4,4-dimethoxy-, ethyl ester
- CAS Number:19255-60-8
- Molecular formula:C8H14O4
- Molecular Weight:174.19
Yield:19255-60-8 90%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 4 h;
Steps:
1
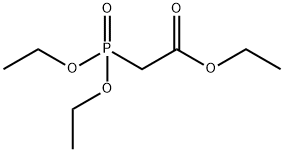
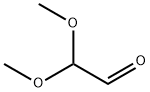
4,4-Dimethoxy-but-2-enoic acid ethyl ester (0037) Add potassium carbonate (16.5 g, 120 mmol) to a solution of dimethoxy acetaldehyde (60% wt. in water) (15 mL, 100 mmol) and triethyl phosphonoacetate (20 mL, 100 mmol) in 210 mL tetrahydrofuran and 30 mL water. Stir the mixture at room temperature for 4 hours. Pour the reaction mixture into diethyl ether (200 mL) and wash with saturated aqueous sodium chloride. Dry the organic phase over sodium sulfate and concentrate under reduced pressure to provide the desired compound as a yellow oil (15.8 g, 90%). 1H-NMR(300 MHz, CDCl3): δ 6.77 (dd, J= 15.9,4.0 Hz, 1H), 6.13 (dd, J= 15.9, 1.4 Hz, 1H), 4.95 (dd, J= 4.0, 1.4 Hz, 1H), 4.22 (q, J=7.1 Hz, 2H), 3.34 (s, 6H), 1.30 (t, J= 7.1 Hz, 3H).
References:
EP2173348,2015,B1 Location in patent:Paragraph 0037