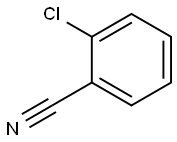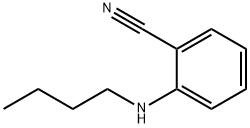
2-(butylamino)benzonitrile synthesis
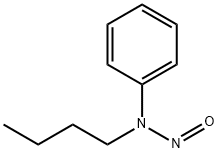
- Product Name:2-(butylamino)benzonitrile
- CAS Number:5589-61-7
- Molecular formula:C11H14N2
- Molecular Weight:174.24
Yield:5589-61-7 97%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);Cs2CO3;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in 1,4-dioxane at 100; for 18 h;Inert atmosphere;Buchwald-Hartwig Coupling;
Steps:
Intermediate 47: 2-(butylamino)benzonitrile
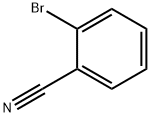
Intermediate 47 was isolated as a yellow oil (416 mg, 97%) according to general procedure 4h, starting from 2- bromobenzonitrile (450 mg) after purification by flash chromatography (CyHex 100% to CyHex/DCM 80:20). M/Z (M+H)+: 175.0. General Procedure 4h: diamine formation - Buchwald coupling To a solution of a halogen derivative (1.0 equiv) in dioxane (0.1 M) was added cesium carbonate (3.0 equiv) and an amine (3.0 equiv). The reaction was sparged with argon for 20 min, then Pd2dba3 (5 mol %) and BINAP (10 mol %) were added. The reaction mixture was heated to 100 °C for 18 h, then it was filtered on Celite. The filtrate was diluted with water and extracted three times with EtOAc. The combined organic layers were washed with brine, dried over sodium sulfate and concentrated to dryness. The crude was purified as detailed hereinafter.
References:
WO2022/64075,2022,A1 Location in patent:Page/Page column 103; 111