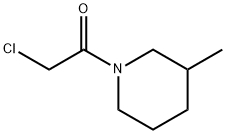
2-CHLORO-1-(3-METHYL-PIPERIDIN-1-YL)-ETHANONE synthesis
- Product Name:2-CHLORO-1-(3-METHYL-PIPERIDIN-1-YL)-ETHANONE
- CAS Number:4593-19-5
- Molecular formula:C8H14ClNO
- Molecular Weight:175.66
Yield:4593-19-5 100%
Reaction Conditions:
in dichloromethane at 0; for 1 h;
Steps:
General Procedure 7 (GP7) - N-acetylation of secondary amine with 2-chloroacetyl chloride:
General procedure: Secondary amine (1.0eq.) was dissolved in DCM (100mL), cooled on an ice-bath, and then 2-chloroacetyl chloride (0.5eq.) was added. The reaction mixture was stirred on ice-bath for 1h, washed with H2O (2×70mL), saturated brine (70mL), dried over Na2SO4, filtered, and the volatiles were evaporatedin vacuo.
References:
Knez, Damijan;Hrast, Martina;Frlan, Rok;Pi?lar, Anja;?akelj, Simon;Kos, Janko;Gobec, Stanislav [Bioorganic Chemistry,2022,vol. 119,art. no. 105581]

107-06-2
411 suppliers
$14.00/25g

79-04-9
350 suppliers
$12.00/5g
53152-98-0
1 suppliers
inquiry

4593-19-5
11 suppliers
$253.00/250mg