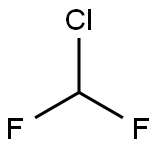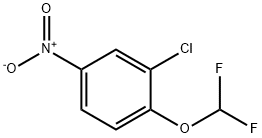
2-Chloro-1-(difluoromethoxy)-4-nitro-benzene synthesis
- Product Name:2-Chloro-1-(difluoromethoxy)-4-nitro-benzene
- CAS Number:40319-63-9
- Molecular formula:C7H4ClF2NO3
- Molecular Weight:223.56
Yield:40319-63-9 69%
Reaction Conditions:
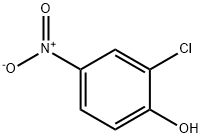
Stage #1: 2-chloro-4-nitrophenolwith potassium hydroxide in methanol;water; for 0.5 h;
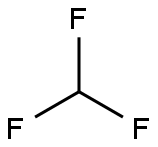
Stage #2: trifluoromethan in methanol;water;acetonitrile at 20; for 3 h;
Steps:
4.3 4.3 Preparation of 1-(difluoromethoxy)-4-nitrobenzene (2a)
General procedure: Using the apparatus previously described for method B
[21]
, potassium hydroxide (2.52 g, 45 mmol, 15 equiv), water (2.52 g), and methanol (1 g) were added to the reaction vessel and the mixture was allowed to stir until the potassium hydroxide was almost completely dissolved.
Then, 4-nitrophenol (0.52 g, 3 mmol) was added and the mixture stirred for 30 min, after which acetonitrile (10 mL) was added via syringe and the mixture stirred at room temperature.
Fluoroform was then bubbled slowly into the mixture for 2 h, after which the resulting mixture was stirred for one additional hour.
After being quenched with water and extracted with ethyl acetate, the ethyl acetate layer was washed with a saturated solution on sodium hydroxide, separated and concentrated.
Additional impurities were removed via column chromatography on silica gel using an 80:20 mixture of hexanes/methylene chloride to give an 83% yield of the liquid product, 1-(difluoromethoxy)-4-nitrobenzene (2a):
References:
Thomoson, Charles S.;Wang, Linhua;Dolbier, William R. [Journal of Fluorine Chemistry,2014,vol. 168,p. 34 - 39]