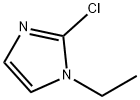
2-Chloro-1-ethyl-1H-imidazole synthesis
- Product Name:2-Chloro-1-ethyl-1H-imidazole
- CAS Number:946061-13-8
- Molecular formula:C5H7ClN2
- Molecular Weight:130.58
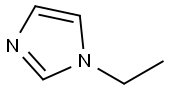
7098-07-9
314 suppliers
$5.00/10g
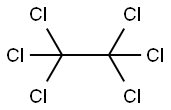
67-72-1
265 suppliers
$11.00/25g

946061-13-8
24 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
Stage #1: N-Ethylimidazolewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: hexachloroethane in tetrahydrofuran;hexane at -78; for 1 h;
Steps:
5.1
(1) 2-Chloro-1-ethyl-1H-imidazole To a solution of 1-ethyl-1H-imidazole (2.2 g) in THF (12 ml), n-BuLi (9.1 ml, 2.64 N in hexane) was added dropwise at -78°C under an argon atmosphere. After stirring at the same temperature for 30 minutes, a solution of hexachloroethane (5.7 g) in THF (12 ml) was added dropwise and stirred at the same temperature for 1 hour. The reaction mixture was diluted with saturated aqueous ammonium chloride, warmed to room temperature, and extracted with AcOEt. After washing with water and saturated aqueous sodium chloride, the organic layer was dried over MgSO4, filtered and then evaporated under reduced pressure to remove the solvent. The resulting crude product was purified by column chromatography (OH-type SiO2, AcOEt/hexane = 0% to 20%) to give the titled compound (2.74 g, colorless oil). 1H NMR (600 MHz, CDCl3) δ ppm: 1.40 (t, J=7.3 Hz, 3H), 3.96 (q, J=7.3 Hz, 2H), 6.88-6.98 (m, 2H)
References:
EP1988081,2008,A1 Location in patent:Page/Page column 36