
2-Chloro-3-hydroxycyclohex-2-en-1-one, 98% synthesis
- Product Name:2-Chloro-3-hydroxycyclohex-2-en-1-one, 98%
- CAS Number:932-23-0
- Molecular formula:C6H7ClO2
- Molecular Weight:146.57

1460-08-8
0 suppliers
inquiry

932-23-0
6 suppliers
inquiry
Yield:932-23-0 10%
Reaction Conditions:
with copper(II) bis(trifluoromethanesulfonate);ammonium chloride in nitromethane; for 20 h;Inert atmosphere;Reflux;
Steps:
2-Chloro-1,3-cyclohexanedione (13a)33
NH4Cl (54 mg, 1.0 mmol) was added to a solution of 12a (69 mg, 0.5 mmol) in MeNO2 (5 mL) at r.t. The reaction mixture was stirred at r.t. for 10 min. Cu(OTf)2 (9 mg, 0.05 mmol) was added and the mixture was stirred at reflux for 20 h. The reaction mixture was cooled to r.t. and the solvent was concentrated. The residue was diluted with H2O (10 mL) and the mixture was extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine, dried, filtered and evaporated under reduced pressure to afford the crude product. Purificationon silica gel (hexanes/EtOAc, 8:1 to 4:1) afforded compound 13a. Yield: 7 mg (10%); colorless oil. 1H NMR (200 MHz, CDCl3): δ = 5.83 (s, 1 H), 2.65-2.59 (m, 4 H), 2.04-1.99 (m, 2 H). HRMS (ESI): m/z [M + 1]+ calcd for C6H8ClO2: 147.0213; found: 147.0218.
References:
Chan, Chieh-Kai;Wang, Heui-Sin;Hsu, Ru-Ting;Chang, Meng-Yang [Synthesis,2017,vol. 49,# 9,p. 2045 - 2056]

504-02-9
554 suppliers
$5.00/10g

932-23-0
6 suppliers
inquiry
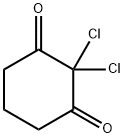
17530-74-4
0 suppliers
inquiry

932-23-0
6 suppliers
inquiry
![Silane, [1-cyclopentene-1,2-diylbis(oxy)]bis[trimethyl-](/CAS/20180601/GIF/6838-66-0.gif)