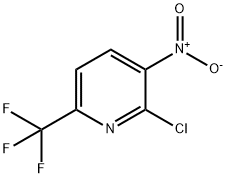
2-Chloro-3-Nitro-6-Trifluoromethyl Pyridine synthesis
- Product Name:2-Chloro-3-Nitro-6-Trifluoromethyl Pyridine
- CAS Number:117519-08-1
- Molecular formula:C6H2ClF3N2O2
- Molecular Weight:226.54
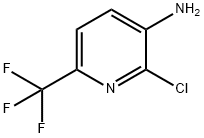
117519-09-2

117519-08-1
General procedure for the synthesis of 2-chloro-3-nitro-6-trifluoromethylpyridine from 2-chloro-6-trifluoromethyl-3-amino-pyridine: To a mixture of fuming sulfuric acid (3.2 mL) and aqueous hydrogen peroxide (50%, 1.6 mL) was slowly added a solution of 3-amino-2-chloro-6-(trifluoromethyl)pyridine in concentrated sulfuric acid (4 mL) at 0°C (304 mg. 1.55 mmol). The reaction mixture was slowly warmed to 25 °C and stirred at this temperature for 20 hours. After completion of the reaction, the mixture was carefully poured into ice water. The reaction mixture was neutralized with aqueous ammonium hydroxide and subsequently extracted with ethyl acetate (5 x 10 mL). The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford the target compound 2-chloro-3-nitro-6-trifluoromethylpyridine (126 mg, 36% yield) as a yellow solid, which could be used in subsequent reactions without further purification.

117519-09-2
188 suppliers
$9.00/250mg

117519-08-1
101 suppliers
$40.00/100mg
Yield:117519-08-1 36%
Reaction Conditions:
Stage #1:3-amino-2-chloro-6-(trifluoromethyl)pyridine with fuming sulphuric acid;dihydrogen peroxide in water at 0 - 25; for 20 h;
Stage #2: with ammonia in water
Steps:
5
To a cooled (0 °C) mixture of fuming sulfuric acid (3.2 mL) and aqueous hydrogen peroxide (50%, 1.6 mL) was added a solution of 3-amino-2-chloro-6-(trifluoromethyl)pyridine (304 mg, 1.55 mmol) in cone, sulfuric acid (4 mL). The reaction was slowly warmed to 25 °C and stirred 20 hours, whereupon the reaction mixture was poured into ice water. The mixture was neutralized with aqueous ammonium hydroxide, and then extracted with ethyl acetate (5 X 10 mL).The organic layers were dried over anhydrous sodium sulfate and concentrated to afford compound 5.1 (126 mg, 36%) as a yellow solid that was used without further purification.
References:
SUNESIS PHARMACEUTICALS, INC. WO2006/65703, 2006, A1 Location in patent:Page/Page column 107