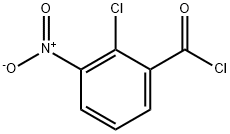
2-chloro-3-nitrobenzoyl chloride synthesis
- Product Name:2-chloro-3-nitrobenzoyl chloride
- CAS Number:34128-16-0
- Molecular formula:C7H3Cl2NO3
- Molecular Weight:220.01
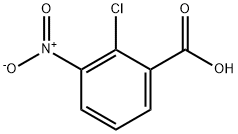
3970-35-2
284 suppliers
$6.00/250mg

34128-16-0
5 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalyl dichloride in tetrahydrofuran at 0 - 20;
Steps:
99
Reference Example 99 Methyl 2-chloro-3-nitrobenzoate To a solution of 2-chloro-3-nitrobenzoic acid (100 g, 0.496 mol) in tetrahydrofuran (1000 mL) was added dropwise oxalyl chloride (46.8 mL, 0.546 mol) at 0° C., and the mixture was stirred at room temperature for 3 hr. To the mixture was added dropwise methanol (300 mL) at 0° C., and the mixture was stirred at room temperature for 14 hr. The mixture was concentrated and the residue was neutralized with saturated aqueous sodium hydrogen carbonate, and extracted with ethyl acetate. The combined organic layer was washed with brine, dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo to give the solid, which was washed with n-hexane to give the title compound as a yellow powder (103 g, 0.478 mol, 96%). 1H NMR (CDCl3) δ 3.98 (s, 3H), 7.47 (t, J=8.7 Hz, 1H), 7.83 (dd, J=1.8 Hz, 8.7 Hz, 1H), 7.94 (dd, J=1.8 Hz, 8.7 Hz, 1H).
References:
US2009/186879,2009,A1 Location in patent:Page/Page column 69