2-Chloro-4-cyclopropoxypyridine synthesis
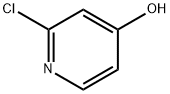
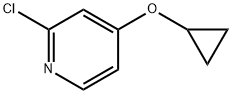
- Product Name:2-Chloro-4-cyclopropoxypyridine
- CAS Number:1209458-93-4
- Molecular formula:C8H8ClNO
- Molecular Weight:169.61
Yield:1209458-93-4 300 mg
Reaction Conditions:
with caesium carbonate;sodium iodide in N,N-dimethyl acetamide at 170 - 180; for 0.833333 h;Microwave irradiation;
Steps:
a (a) 2-chloro-4-cyclopropoxypyridine
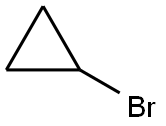
To a solution of 2-chloropyridin-4-ol (1 g, 7.75 mmol) in DMA (10 ml) was added bromocyclopropane (2.8 g, 23.2 mmol), Nal (1.16 g, 7.75 mmol) and CS2CO3 (5 g, 15.5 mmol). The mixture was stirred at MW 170 °C for 20 minutes, and then MW 180 °C for 30 minutes. The reaction mixture was extracted with EA. The organic layer was dried and concentrated. The residue was purified by flash column chromatography to give 300 mg of 2-chloro-4-cyclopropoxypyridine. 1H NMR (400MHz, CDC13) δ = 8.19 (d, J=5.8 Hz, IH), 7.02 (d, J=2.0 Hz, IH), 6.87 (dd, J=2.0, 5.8 Hz, IH), 3.80 (tt, J=3.0, 6.0 Hz, IH), 0.91 - 0.75 (m, 4H).
References:
WO2014/114185,2014,A1 Location in patent:Page/Page column 162