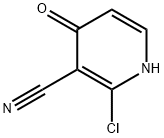
2-CHLORO-4-ETHOXYNICOTINONITRILE synthesis
- Product Name:2-CHLORO-4-ETHOXYNICOTINONITRILE
- CAS Number:98645-45-5
- Molecular formula:C8H7ClN2O
- Molecular Weight:182.61
Yield:98645-45-5 98%
Reaction Conditions:
Stage #1: 1,1-dicyano-4-(N,N-dimethylamino)-2-methoxy-1,3-butadiene;1,1-Dicyan-4-(N,N-dimethylamino)-2-ethoxy-1,3-butadienwith hydrogenchloride in methanol at 0;
Stage #2: with potassium carbonate in dichloromethane;water;
Steps:
41.41B
Example 41B; 2-Chloro-3 -cyano-4-ethoxypyridine; :To a slurry of a mixture of 2-(3-dimethylamino-l-ethoxy-allylidene)malononitrile and 2-(3-dimethylamino-l-methoxyallylidene)malono-nitrile (85.0 g, 445 mmol) from step 1, in methanol (1200 mL), HCl gas was introduced gently at 0 0C. The reaction mixture became homogeneous in about 1 hr, and was allowed to stir under constant HCl flow for additional 14.5 hours at 0 0C. N2 was bubled though the reaction mixture for over night, and all the solvent was removed. The residue solid was re-dissolved in CH2Cl2, and washed with water/KaCCVwater. Organic layer was separated and dried over Na2SO4. Removal of salt and solvent gave a pure product (113.0 g, 98%). IH NMR (CDC13, 300 MHz): δ = 7.96 ppm (d, J= 6.4 Hz, IH); 6.59 (d, J= 6.4 Hz, IH); 3.30 (s, 6H). MS (ESI, M+l): 181.9.
References:
WO2006/81072,2006,A1 Location in patent:Page/Page column 48
180995-12-4
121 suppliers
$24.19/250mg

64-17-5
734 suppliers
$10.00/50g

98645-45-5
7 suppliers
inquiry
![Propanedinitrile, 2-[3-(dimethylamino)-1-ethoxy-2-propen-1-ylidene]-](/CAS/20210305/GIF/98645-41-1.gif)
98645-41-1
1 suppliers
inquiry

98645-45-5
7 suppliers
inquiry
4637-24-5
616 suppliers
$5.00/25g
5417-82-3
119 suppliers
$9.00/1g

98645-43-3
129 suppliers
$22.00/250mg

98645-45-5
7 suppliers
inquiry
![2-[3-(DIMETHYLAMINO)-1-METHOXY-2-PROPENYLIDENE]MALONONITRILE](/CAS/GIF/95689-38-6.gif)