
(2-chloro-4-isopropoxyphenyl)methanol synthesis
- Product Name:(2-chloro-4-isopropoxyphenyl)methanol
- CAS Number:219764-66-6
- Molecular formula:C10H13ClO2
- Molecular Weight:200.66
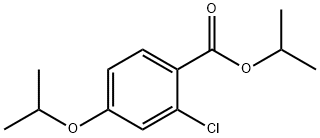
219764-65-5
1 suppliers
inquiry

219764-66-6
13 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: isopropyl 2-chloro-4-isopropoxybenzoatewith lithium aluminium tetrahydride in diethyl ether; for 2 h;
Stage #2: with sodium hydroxide in diethyl ether;water at 20; for 0.5 h;
Steps:
76.2 Preparation Example 76-2 2-Chloro-4-isopropoxybenzyl alcohol
Preparation Example 76-2 2-Chloro-4-isopropoxybenzyl alcohol lithium aluminum hydride (100 mg) was added to a solution of isopropyl 2-chloro-4-isopropoxybenzoate (675 mg) in ether (6.8 ml) and the mixture was stirred for 2 hr.. To the reaction mixture were dropwise added successively water (0.8 ml), a 1N aqueous sodium hydroxide solution (0.8 ml) and water (2.4 ml) under ice-cooing, and the mixture was stirred for 30 min at room temperature.. Then ether and water were added and the organic layer was separated.. The resulting product was extracted with ether from the aqueous layer.. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate.. The solvent was evaporated to give the objective compound (513 mg) as a brown oil. 1H-NMR(CDCl3): 1.32(6H, d, J=6 Hz), 1.97(1H, t, J=6 Hz), 4.52(1H, m), 4.70(2H, d, J=6 Hz), 6.78(1H, dd, J=2 Hz), 6.92(1H, d, J=2 Hz), 7.33(1H, d, J=8 Hz).
References:
US6348474,2002,B1 Location in patent:Page column 112
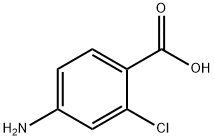
2457-76-3
293 suppliers
$5.00/1g

219764-66-6
13 suppliers
inquiry
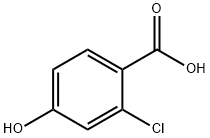
56363-84-9
172 suppliers
$14.14/250mgs:

219764-66-6
13 suppliers
inquiry