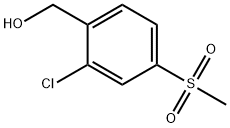
(2-CHLORO-4-METHANESULFONYL-PHENYL)-METHANOL synthesis
- Product Name:(2-CHLORO-4-METHANESULFONYL-PHENYL)-METHANOL
- CAS Number:181300-40-3
- Molecular formula:C8H9ClO3S
- Molecular Weight:220.67
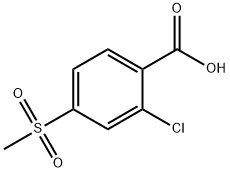
53250-83-2
238 suppliers
$13.00/1g

181300-40-3
21 suppliers
$159.06/1gm:
Yield:181300-40-3 53%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0;Inert atmosphere;
Steps:
62
Lithium tetrahydroaluminum (97mg, 2.56mmol) was placed in tetrahydrofuran (5mL) and placed in an ice bath to cool under the protection of nitrogen. After dissolving 2-chloro-4-(methylsulfonyl)benzoic acid (500mg, 2.13mmol) in anhydrous THF (5mL), it was added dropwise to lithium tetrahydroaluminum, and the mixture was stirred under ice cooling until the reaction was completed. Add ice water (100 μL) and sodium hydroxide (200 mg), stir for 5 min, then add water (300 μL), stir for 10 min, filter through Celite, and rinse the filter cake with methanol. The solvent in the filtrate was evaporated under reduced pressure, water (10mL) was added, and the mixture was extracted with ethyl acetate (10mL×3). The combined organic phase was washed with water (10mL) and saturated aqueous sodium chloride solution (10mL) successively, and anhydrous Dry with sodium sulfate. Dry loading, and silica gel column chromatography (dichloromethane/methanol=200:1) to obtain compound B7 as a pale yellow solid (250 mg, yield 53%).
References:
CN112608320, 2021, A Location in patent:Paragraph 0369-0373; 0374

101349-95-5
29 suppliers
$60.00/100mg

181300-40-3
21 suppliers
$159.06/1gm:
84194-36-5
337 suppliers
$9.00/1g

181300-40-3
21 suppliers
$159.06/1gm: