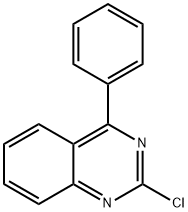
2-CHLORO-4-PHENYLQUINAZOLINE synthesis
- Product Name:2-CHLORO-4-PHENYLQUINAZOLINE
- CAS Number:29874-83-7
- Molecular formula:C14H9ClN2
- Molecular Weight:240.69
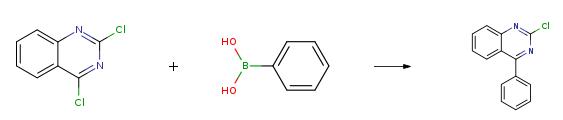
A 2 L flask was charged with 35.0 g (175.8 mmol) of 2,4-dichloroquinoline,22.5 g (184.6 mmol) of phenylboronic acid, 6.1 g (5.3 mmol) of tetrakis(triphenylphosphine)palladium(0) were added to 800 mL of tetrahydrofuran and 400 mL of water, and the mixture was heated to 60 ° C for 12 hours under a nitrogen stream. . The resulting mixture was added to methanol (3000 mL), and the crystallized solid was filtered and dissolved in toluene, filtered through silica gel / celite, and the organic solvent was removed in an appropriate amount, and then recrystallization from methanol gave Intermediate I-A52-1 (36.8 g, 87% yield).
Yield:29874-83-7 87%
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium;potassium carbonate in tetrahydrofuran;lithium hydroxide monohydrate at 60; for 12 h;Inert atmosphere;
Steps:
4 Synthesis of intermediate I-A52-1
A 2 L flask was charged with 35.0 g (175.8 mmol) of 2,4-dichloroquinoline,22.5 g (184.6 mmol) of phenylboronic acid, 6.1 g (5.3 mmol) of tetrakis(triphenylphosphine)palladium(0) were added to 800 mL of tetrahydrofuran and 400 mL of water, and the mixture was heated to 60 ° C for 12 hours under a nitrogen stream. . The resulting mixture was added to methanol (3000 mL), and the crystallized solid was filtered and dissolved in toluene, filtered through silica gel / celite, and the organic solvent was removed in an appropriate amount, and then recrystallization from methanol gave Intermediate I-A52-1 (36.8 g, 87% yield).
References:
KR2017/10582,2017,A Location in patent:Paragraph 0251-0254
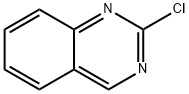
6141-13-5
130 suppliers
$22.00/100mg

108-86-1
497 suppliers
$10.00/5g

29874-83-7
187 suppliers
$10.00/1g
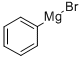
100-58-3
185 suppliers
$18.00/10ml
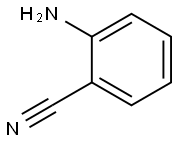
1885-29-6
442 suppliers
$5.00/1g

29874-83-7
187 suppliers
$10.00/1g
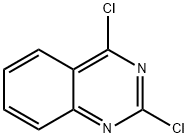
607-68-1
312 suppliers
$10.00/1g
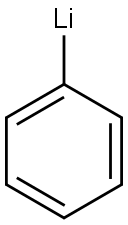
591-51-5
126 suppliers
$123.00/50ml

29874-83-7
187 suppliers
$10.00/1g
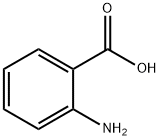
118-92-3
4 suppliers
$23.00/250g

29874-83-7
187 suppliers
$10.00/1g
