(2-chloro-5-iodophenyl)(4-methoxyphenyl)methanone synthesis
- Product Name:(2-chloro-5-iodophenyl)(4-methoxyphenyl)methanone
- CAS Number:1459754-39-2
- Molecular formula:C14H10ClIO2
- Molecular Weight:372.59
19094-56-5
520 suppliers
$6.00/5g
100-66-3
511 suppliers
$10.00/5G

1459754-39-2
14 suppliers
inquiry
Yield:1459754-39-2 91%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 20; for 3 h;Cooling with ice;
Steps:
4.2 (2) Preparation of (2-chloro-5-iodophenyl) (4-methoxyphenyl) methanone
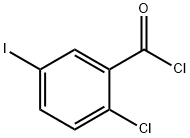
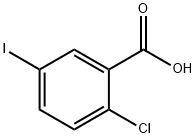
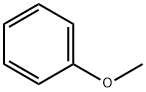
2-Chloro-5-iodobenzoyl chloride (10.7 g, 35.5 mmol) was dissolved in methylene chloride (200 mL). The resulting mixture was cooled in an ice-water bath. To this mixture was added aluminum trichloride (10.4 g, 78.2 mmol) followed by the dropwise addition of a solution of anisole (4.2 g, 38.9 mmol) in methylene chloride (50 mL). After completion of the dropwise addition, the resulting mixture was warmed to room temperature and stirred for 3 hours. The reaction mixture was poured into ice water and quenched. 3 mol / L hydrochloric acid was added to the reaction mixture. The product mixture was separated into an aqueous phase and an organic phase. The aqueous phase was extracted with methylene chloride (150 mL x 2). The organic phases were combined, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography (ethyl acetate: petroleum ether = 0-1: 100) to give 12.0 g of product in 91% yield.
References:
KR2015/81220,2015,A Location in patent:Paragraph 0225-0227

19094-56-5
520 suppliers
$6.00/5g

1459754-39-2
14 suppliers
inquiry