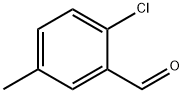
2-Chloro-5-Methylbenzaldehyde synthesis
- Product Name:2-Chloro-5-Methylbenzaldehyde
- CAS Number:14966-09-7
- Molecular formula:C8H7ClO
- Molecular Weight:154.59
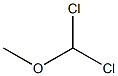
4885-02-3
181 suppliers
$10.00/1g
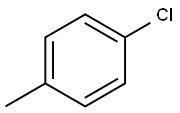
106-43-4
328 suppliers
$14.00/5g
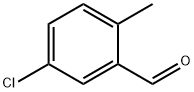
58966-34-0
71 suppliers
$88.00/1g

14966-09-7
38 suppliers
$32.00/250mg
Yield:-
Reaction Conditions:
with titanium tetrachloride in dichloromethane at 20; for 15 h;
Steps:
182 Reference Example 182
To a solution of 4-chlorotoluene (25.0 g) and dichloromethylmethylether (45.4 g) in dichloromethane (160 ml) was added dropwise at room temperature a solution of titanium tetrachloride (74.9 g) in dichloromethane (40 ml), and the mixture was stirred at the same temperature for 15 hours. The reaction solution was poured into ice, and the organic layer was washed with water, sodium hydrogen carbonate solution, water and saturated brine, and dried with magnesium sulfate. Under reduced pressure, the solvent was evaporated, and the residue was subjected to silica gel column chromatography (ethyl acetate-hexane) to give oil of crude 2-chloro-5-methylbenzaldehyde (18.3 g) and 5-chloro-2-methylbenzaldehyde (4.1 g), respectively. To a mixture of acetone (160 ml), sodium hydroxide (2.6 g) and water (160 ml) was added dropwise at 0° C. a solution of crude 2-chloro-5-methylbenzaldehyde (18.3 g) in acetone (30 ml), and the mixture was stirred at the same temperature for 1 hour. Under reduced pressure, acetone was evaporated, and the residue was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and concentrated under reduced pressure to give 4-(2-chloro-5-methylphenyl)-3-buten-2-one (18.9 g) as oil. To a solution of 20% sodium ethoxide in ethanol (4.3 g) was added at room temperature diethyl malonate (10.1 g), and then added little by little 4-(2-chloro-5-methylphenyl)-3-buten-2-one (18.9 g). The mixture was stirred at room temperature for 30 minutes, refluxed for 2 hours and cooled. The solvent was evaporated, and to the residue was added water. The aqueous layer was washed with ethyl acetate and concentrated. To the residue was added 2M sodium hydroxide (33 ml), and the mixture was refluxed for 2 hours and cooled. To the mixture was added 2.5M sulfuric acid (33 ml) for 15 minutes, and the mixture was refluxed for 30 minutes and cooled. Precipitated crystals were filtered and washed with water and isopropylether to give 5-(2-chloro-5-methylphenyl)cyclohexane-1,3-dione (7.8 g) as colorless crystals. mp 186-188° C. 1H-NMR(CDCl3) δ: 2.33 (3H, s), 2.38-2.72 (4H, m), 3.2-5.4 (1H, br), 3.73-3.93 (1H, m), 5.55 (1H, s), 7.01 (1H, d, J=8 Hz), 7.03 (1H, s), 7.26 (1H, d, J=8 Hz). To a mixture of acetone (80 ml), sodium hydroxide (1.2 g) and water (80 ml) was added dropwise at 0° C. a solution of 5-chloro-2-methylbenzaldehyde (4.1 g) in acetone (10 ml), and the mixture was stirred at the same temperature for 1 hour. Under reduced pressure, acetone was evaporated, and the residue was extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and concentrated under reduced pressure to give 4-(5-chloro-2-methylphenyl)-3-buten-2-one (5.5 g) as oil. To a solution of 20% sodium ethoxide in ethanol (9.5 g) was added at room temperature diethyl malonate (4.5 g) and then added little by little 4-(5-chloro-2-methylphenyl)-3-buten-2-one (5.5 g). The mixture was stirred at room temperature for 30 minutes, refluxed for 2 hours and cooled. The solvent was evaporated, and to the residue was added water. The aqueous layer was washed with ethyl acetate and concentrated. To the residue was added 2M sodium hydroxide (15 ml), and the mixture was refluxed for 2 hours and cooled. To the mixture was added 2.5M sulfuric acid (15 ml) for 15 minutes, and the mixture was refluxed for 30 minutes and cooled. Precipitated crystals were filtered and washed with water and isopropylether to give 5-(5-chloro-2-methylphenyl)cyclohexane-1,3-dione (2.9 g) as colorless crystals. mp 180-181° C. 1H-NMR(CDCl3-DMSO-d6) δ: 2.31 (3H, s), 2.35-2.84 (4H, m), 3.37-3.73 (1H, m), 5.56 (1H, s), 6.9-7.43 (1H, br), 7.08-7.26 (3H, m).
References:
Takeda Chemical Industries, Ltd. US6350749, 2002, B1 Location in patent:Page column 135 - 136