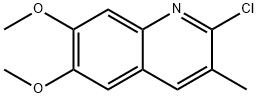
2-Chloro-6,7-dimethoxy-3-methyl-quinoline synthesis
- Product Name:2-Chloro-6,7-dimethoxy-3-methyl-quinoline
- CAS Number:577967-81-8
- Molecular formula:C12H12ClNO2
- Molecular Weight:237.68
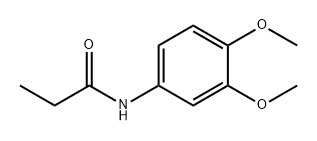
63914-26-1
1 suppliers
inquiry

577967-81-8
21 suppliers
$158.00/100mg
Yield:577967-81-8 67%
Reaction Conditions:
with N,N-dimethyl-formamide;trichlorophosphate in N,N-dimethyl-formamide at 20 - 75; for 2 h;
Steps:
113.B Example 113; General Procedure (B); 2-(4-Cyclopropylpiperazin-1-yl)-6,7-dimethoxy-3-methylquinoline hydrochloride
The required 2-chloro-6,7-dimethoxy-3-methylquinoline was prepared according to the procedure published in Tetrahedron Letters 1979, 4885, in the following way: [0812] To a solution of 3,4-dimethoxyaniline (4.70 g, 30.7 mmol) in dichloromethane (50 ml) were added pyridine (8.0 ml, 3 equivalents) and then dropwise propionyl chloride (3.5 ml, 40.5 mmol). After stirring at room temperature for 1 h and 50 min the mixture was poured into a mixture of water (200 ml) and concentrated hydrochloric acid (8 ml). The phases were separated, and the aqueous phase was extracted once with dichloromethane. Washing of the combined organic phases with brine, drying with magnesium sulfate, and concentration yielded 6.89 g of an oil which crystallized after a few minutes. Recrystallization from a mixture of ethyl acetate and heptane yielded 3.60 g (49%) of N-(3,4-dimethoxyphenyl)propionamide as dark crystals. [0813] This anilide (2.1 g, 10.0 mmol) was mixed with DMF (1.1 ml, 15 mmol), and to this mixture POCl3 (6.5 ml, 70 mmol) was dropwise added at room temperature. When the addition is finished the mixture is stirred at 75° C. for 2 h. The mixture was poured into ice-water (100 ml), stirred for 30 min, and filtered. The solid was stripped with toluene and acetonitrile, to yield 1.60 g (67%) of 2-chloro-6,7-dimethoxy-3-methylquinoline as a solid. This product was treated with 1-cyclopropylpiperazine as described in General Procedure (B) to yield the title compound. [0814] 1H NMR (DMSO-d6) δ0.83 (m, 2H), 1.23 (m, 2H), 2.42 (s, 3H), 2.94 (m, 1H), 3.40-4.50 (m, 8H), 3.88 (s, 3H), 3.91 (s, 3H), 7.29 (s, 1H), 7.48 (br s, 1H), 8.12 (br s, 1H), 11.24 (br s, 1H); HPLC-MS: m/z 328 (MH+); Rt=1.63 min.
References:
US2003/236259,2003,A1 Location in patent:Page 51