
2-Chloro-6-hydroxyquinoline synthesis
- Product Name:2-Chloro-6-hydroxyquinoline
- CAS Number:577967-89-6
- Molecular formula:C9H6ClNO
- Molecular Weight:179.6

13676-02-3
103 suppliers
$30.00/250mg

577967-89-6
83 suppliers
$15.00/250mg
Yield: 93%
Reaction Conditions:
with boron tribromide in dichloromethane at 0 - 20; for 15 h;
Steps:
General procedure: To a cooled solution of aryl methyl ether ( 1.0 equiv.) in DCM (10 mL) was added BBr3 (5.0 equiv.) slowly. The resulting mixture was stirred at 0 C and then warmed gradually to room temperature, and. stirred at room temperature for 15 hrs. After the reaction was completed, the reaction was quenched with NaHCO3 solution (50 mL) and extracted with DCM (4 × 10 mL). The combined organic layers were washed with H2O (3 × 10 mL), dried (Na2SO4) and concentrated in vacuo to afford the expected phenols. 2-chloroquinolin-6-ol: DHK-6-71 was prepared using general procedure G. Reaction was performed on a 2 g scale. DHK-6-71 was isolated as yellow solid. (1.72 g. 93%).
References:
Eli Lilly and Company;Cashion, D.K;chen, G.;Kasi, D;kolb, C;liu, C;sinha, A;Szardenings, A.k;wang, E;yu, C;zhang, W;Gangadharmath, Umesh B;Walsh, J.C CN102985411, 2016, B Location in patent:Paragraph 0906-0908
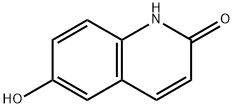
19315-93-6
221 suppliers
$7.00/1g

577967-89-6
83 suppliers
$15.00/250mg
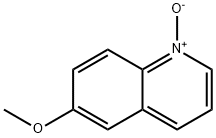
6563-13-9
61 suppliers
$39.70/1g

577967-89-6
83 suppliers
$15.00/250mg
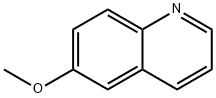
5263-87-6
301 suppliers
$15.00/5g

577967-89-6
83 suppliers
$15.00/250mg
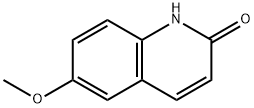
13676-00-1
62 suppliers
$285.00/100mg

577967-89-6
83 suppliers
$15.00/250mg