
2-CHLORO-6-METHOXY-3-QUINOLINECARBALDEHYDE OXIME synthesis
- Product Name:2-CHLORO-6-METHOXY-3-QUINOLINECARBALDEHYDE OXIME
- CAS Number:93299-50-4
- Molecular formula:C11H9ClN2O2
- Molecular Weight:236.65
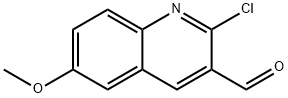
73568-29-3
90 suppliers
$15.00/1g

93299-50-4
26 suppliers
$232.00/500mg
Yield:-
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydroxide in ethanol at 20; for 0.75 h;
Steps:
General procedure for the synthesis of 3-(2-chloroquinolin-3-yl)-5-phenylisoxazol (3a-j)
General procedure: The corresponding precursor, 2-chloroquinolin-3-carbaldehyde (2a-j) (1.0 equiv.) was added to a hydroxylamine solution (1.1 equiv.) in ethanol. Then NaOH was added (1.1 equiv.) to the reaction mixture, which was then stirred at room temperature for 45 minutes. The oxyme formation was corroborated by thin-layer chromatographic (TLC) analysis. Chloramine-T trihydrate (1.1 equiv.) was added, followed by CuSO4°5H2O (0.03 equiv.) and Cu (0.01 equiv.). When a change of color was observed, 10 mL of THF followed by phenylacetylene (1.1 equiv.) were added to the solution and stirred for 8 hours. Once finished, the reaction was filtered to remove copper salts, washed with water, dried with anhydrous Na2SO4 and recrystallized from ethanol. The derivatives which could not be purified using recrystallization techniques were purified using column chromatography in silica gel, using an 8:2 Hexane/EtOAc mixture as the mobile phase, except the derivative 2d, which was purified using only dichloromethane as the mobile phase.
References:
Ferna?ndez-Galleguillos, Carlos;Saavedra, Luis A.;Gutierrez, Margarita [Journal of the Brazilian Chemical Society,2014,vol. 25,# 2,p. 365 - 371]
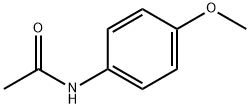
51-66-1
221 suppliers
$12.00/1mg

93299-50-4
26 suppliers
$232.00/500mg
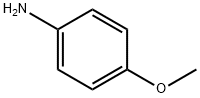
104-94-9
481 suppliers
$9.00/1g

93299-50-4
26 suppliers
$232.00/500mg