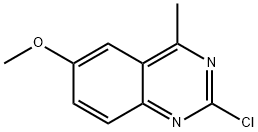
2-chloro-6-methoxy-4-methylquinazoline synthesis
- Product Name:2-chloro-6-methoxy-4-methylquinazoline
- CAS Number:952434-87-6
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64

92210-45-2
1 suppliers
inquiry

952434-87-6
7 suppliers
inquiry
Yield:952434-87-6 51.6%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate in toluene at 90; for 4 h;
Steps:
Synthesis of 2-chloro-6-methoxy-4-methyl-quinazoline
Synthesis of 2-chloro-6-methoxy-4-methyl-quinazoline To a solution of compound 7 (10 g, 0.081 mol) in 20 mL of 1 ,2-dichloroethanewas added dropwise BCI3 (200 mL, 1 .0 Min toluene) at 0°C. After being stirred at 0°C for 30 min, 50 mL of CH3CN was added, then the mixture was stirred at80°C for 12 hours. The resulting mixture was concentrated in vacuum, theresidue was dissolved in 100 mL of HCI (2M) at 0°C and the mixture was stirredat 80°C for 30 min, the reaction mixture was neutralized with aqueous Na2CO3till pH = 7, and extracted with CH2CI2, the organic layers were concentrated invacuum, the residue was purified by chromatography on silica gel (PE / EtOAc =30: 1 ) to afford compound 7a (0.9 g, yield: 6.7%). m/z = 166 [M + H]+ .To a solution of the compound 7a (0.9g, 5.45 mmol) in 20 mL of THF was added DMAP (0.73 g, 6.0 mmol) and CI3CCOCI(0.66 mL, 6.0 mmol) at 0°C, then the mixture was stirred at room temperaturefor 2 hours. The resulting mixture was diluted with ice water (50 mL),extracted with EtOAc (50 mL χ 3), the organiclayers were concentrated in vacuum to give compound 7b (1 .6 g, yield: 94.6%),which was used for next step without further purification. To a solution of the compound 7b (1 .7 g, 5.5 mmol)in 20 mL of DMSO was added CH3COONH (1 .7 g, 22 mmol),the mixture was stirred at room temperature for 20 hours. The resulting mixturewas diluted with H2O (200 ml_),extracted with EtOAc (50 ml_ χ 3), the organic layers were concentrated invacuum to give 6-methoxy-4-methyl-1 H-quinazolin-2-one (0.6 g, yield: 60%),which was used for next step without further purification, m/z = 191 [M + H]+ .To a solutionof the compound 6-methoxy-4-methyl-1 H-quinazolin-2-one (200 mg, 1 .05 mmol) in15 ml_ of toluene was added POCI3 (0.2 ml_, 2.1 mmol) and DIPEA (0.38 ml_, 2.1mmol), then the mixture was stirred at 90°C for 4 hours. The resulting mixturewas concentrated in vacuum, the residue was dissolved with Et2O(50 ml_), the organic layer was collected and dried to give2-chloro-6-methoxy-4-methyl-quinazoline (120 mg, yield: 51 .6%). m/z = 209 [M +H]+
References:
WO2013/50527,2013,A1 Location in patent:Page/Page column 50; 51
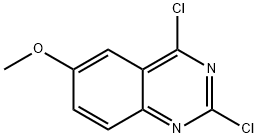
105763-77-7
131 suppliers
$34.00/100mg
5158-46-3
27 suppliers
$45.00/250mg

952434-87-6
7 suppliers
inquiry

105763-77-7
131 suppliers
$34.00/100mg
676-58-4
275 suppliers
$15.00/25ml

952434-87-6
7 suppliers
inquiry

32618-84-1
55 suppliers
inquiry

952434-87-6
7 suppliers
inquiry
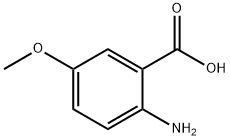
6705-03-9
256 suppliers
$9.00/1g

952434-87-6
7 suppliers
inquiry