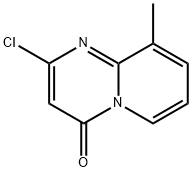
2-chloro-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-one synthesis
- Product Name:2-chloro-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
- CAS Number:17326-22-6
- Molecular formula:C9H7ClN2O
- Molecular Weight:194.62
![4H-Pyrido[1,2-a]pyriMidin-4-one,2-hydroxy-9-Methyl-](/CAS/GIF/17326-09-9.gif)
17326-09-9
28 suppliers
$27.00/250mg
![2-chloro-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/20180629/GIF/17326-22-6.gif)
17326-22-6
16 suppliers
$90.00/50mg
Yield:17326-22-6 77%
Reaction Conditions:
Stage #1: 2-hydroxy-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-onewith trichlorophosphate at 100; for 3 h;Cooling with ice;
Stage #2: with sodium hydroxide in water;
Steps:
1.2
Step 2. 2-Chloro-9-methyl-4H-pyrido[1 ,2-a]pyrimidin-4-oneA mixture of 2-hydroxy-9-methyl-4 - -pyrido[1 ,2-a]pyrimidin-4-one (17.0 g, 96 mmol) and POCI3 (45 ml_, 482 mmol) was stirred at 100 °C for 3 h. The mixture was dropped into stirring water at RT (intermittently cooled with an ice-water bath to maintain the temperature). The mixture was neutralized with 20% aq. NaOH. The suspension was filtered, and the solid was washed with water to give the titled compound (14.5 g, 77%) as a pale brown solid.1H NMR (CDCI3) δ (ppm) 8.92 (d, J = 7.2 Hz, 1 H), 7.67 (d, J = 6.8 Hz, 1 H), 7.14-7.08 (m, 1 H), 6.44 (s, 1 H), 2.57 (s, 3H)MS (ES+) 195, 197
References:
WO2012/38850,2012,A1 Location in patent:Page/Page column 29-30
1603-40-3
475 suppliers
$6.00/25g
![2-chloro-9-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](/CAS/20180629/GIF/17326-22-6.gif)
17326-22-6
16 suppliers
$90.00/50mg