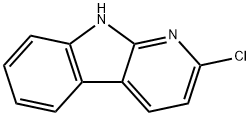
2-CHLORO-9H-PYRIDO[2,3-B]INDOLE synthesis
- Product Name:2-CHLORO-9H-PYRIDO[2,3-B]INDOLE
- CAS Number:26869-12-5
- Molecular formula:C11H7ClN2
- Molecular Weight:202.64
![Acetamide, N-[2-(2,6-dichloro-3-pyridinyl)phenyl]-](/CAS/20210305/GIF/148493-40-7.gif)
148493-40-7
0 suppliers
inquiry
![2-CHLORO-9H-PYRIDO[2,3-B]INDOLE](/CAS/20180601/GIF/26869-12-5.gif)
26869-12-5
14 suppliers
inquiry
Yield: 93%
Reaction Conditions:
with sodium hydride;potassium carbonate in N,N-dimethyl-formamide;mineral oil at 100; for 3 h;Inert atmosphere;Time;
Steps:
3; 36.3 Step 3: 2-chloro-9H-pyrido[2,3-b]indole
To a mixture of N-[2-(2,6-dichloro-3-pyridyl)phenyl]acetamide (1.7 g, 6.05 mmol) and K2CO3 (5.01 g, 36.3 mmol) in DMF (30 mL) was added NaH (483.70 mg, 12.09 mmol, 60% purity) under nitrogen protection. Then the mixture was stirred at 100° C. for 3 hours. On completion, the reaction mixture was poured into ice water (200 mL) and stirred for half an hour. Then the suspension was filtered to get the solid and dried in vacuo to give the title compound (1.2 g, 93% yield) as a yellow solid. LC-MS (ESI, m/z): [M+1]+=203.4.
References:
Kymera Therapeutics, Inc.;Ji, Nan;Kluge, Arthur F.;Weiss, Matthew M.;Zhang, Yi US2020/10468, 2020, A1 Location in patent:Paragraph 0725; 0728; 0885; 0888
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2-CHLORO-9H-PYRIDO[2,3-B]INDOLE](/CAS/20180601/GIF/26869-12-5.gif)
26869-12-5
14 suppliers
inquiry
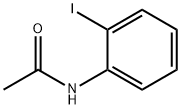
19591-17-4
52 suppliers
$55.00/100mg
![2-CHLORO-9H-PYRIDO[2,3-B]INDOLE](/CAS/20180601/GIF/26869-12-5.gif)
26869-12-5
14 suppliers
inquiry
![9H-Pyrido[2,3-b]indole, 2-chloro-, 1-oxide](/CAS/20210111/GIF/30151-86-1.gif)
30151-86-1
0 suppliers
inquiry
![2-CHLORO-9H-PYRIDO[2,3-B]INDOLE](/CAS/20180601/GIF/26869-12-5.gif)
26869-12-5
14 suppliers
inquiry
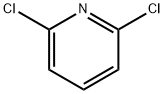
2402-78-0
512 suppliers
$6.00/25g
![2-CHLORO-9H-PYRIDO[2,3-B]INDOLE](/CAS/20180601/GIF/26869-12-5.gif)
26869-12-5
14 suppliers
inquiry