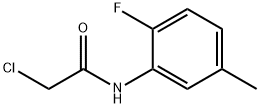
2-chloro-N-(2-fluoro-5-methylphenyl)acetamide synthesis
- Product Name:2-chloro-N-(2-fluoro-5-methylphenyl)acetamide
- CAS Number:630119-82-3
- Molecular formula:C9H9ClFNO
- Molecular Weight:201.63
Yield:630119-82-3 100%
Reaction Conditions:
with sodium hydrogencarbonate in ethyl acetate at 0 - 20; for 1.66667 h;
Steps:
20 Preparation of 2-chloro-N-(2-fluoro-5-methylphenyl)acetamide (C52)
Example 20
Preparation of 2-chloro-N-(2-fluoro-5-methylphenyl)acetamide (C52)
To a reaction flask were added 2-fluoro-5-methylaniline (3.00 g, 24.0 mmol) and ethyl acetate (24.0 mL).
The reaction mixture was cooled to 0° C. Sodium bicarbonate (4.03 g, 47.9 mmol) was added, followed by dropwise addition of chloroacetyl chloride (2.30 mL, 28.8 mmol) over 4 minutes.
The reaction mixture was allowed to stir at 0° C. for 10 minutes, then was allowed to warm to room temperature and was further stirred for 90 minutes.
Water (15 mL) was added to the reaction mixture, and the phases were separated.
The organic layers were washed with brine (20 mL), dried over magnesium sulfate, filtered, and concentrated to afford the title compound (4.83 g, 100%): 1H NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 8.08 (dd, J=7.5, 2.1 Hz, 1H), 7.00 (dd, J=10.7, 8.4 Hz, 1H), 6.90 (dddd, J=8.3, 5.0, 2.2, 0.8 Hz, 1H), 4.21 (s, 2H), 2.34 (d, J=0.9 Hz, 3H); 19F NMR (376 MHz, CDCl3) δ -135.59; 13C NMR (126 MHz, CDCl3) δ 163.83, 151.92, 150.00, 134.42, 125.79, 121.94, 114.64, 42.92, 21.08; ESIMS m/z 201 ([M+H]+).
References:
US2017/64962,2017,A1 Location in patent:Paragraph 0561; 0562
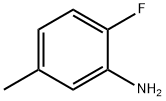
452-84-6
250 suppliers
$6.00/5g

630119-82-3
11 suppliers
inquiry
