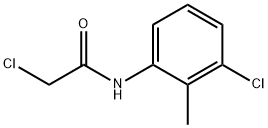
2-CHLORO-N-(3-CHLORO-2-METHYLPHENYL)ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(3-CHLORO-2-METHYLPHENYL)ACETAMIDE
- CAS Number:99585-94-1
- Molecular formula:C9H9Cl2NO
- Molecular Weight:218.08
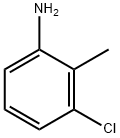
87-60-5
411 suppliers
$8.00/25g

79-04-9
354 suppliers
$12.00/5g

99585-94-1
20 suppliers
$60.00/100mg
Yield:99585-94-1 97%
Reaction Conditions:
with triethylamine in ethyl acetate at 0 - 20; for 2 h;Inert atmosphere;
Steps:
General Procedure II
General procedure: To aniline (1.0 eq.) in ethyl acetate, 2-chloroacetyl chloride (1.1 eq.) was added. The reaction was then cooled to 0C and under N2. Triethylamine (1.1 eq.) was added dropwise, and the reaction was warmed to room temperature and stirred for 2 hours. The solvent was removed in vacuo and taken back up into ethyl acetate and water. Product,10, was purified by flash chromatography 0-40% EtOAc:hexanes.
References:
Tolentino, Kirsten T.;Mashinson, Viktoriya;Vadukoot, Anish K.;Hopkins, Corey R. [Bioorganic and Medicinal Chemistry Letters,2022,vol. 61,art. no. 128615] Location in patent:supporting information

87-60-5
411 suppliers
$8.00/25g

99585-94-1
20 suppliers
$60.00/100mg