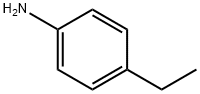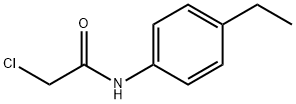
2-CHLORO-N-(4-ETHYLPHENYL)ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(4-ETHYLPHENYL)ACETAMIDE
- CAS Number:20172-36-5
- Molecular formula:C10H12ClNO
- Molecular Weight:197.66
Yield: 92%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
General procedure for the preparation of α-chloro-N-arylacetamide (2a-i)
General procedure: To the mixture of substituted aromatic amine (0.020 mol)and Et3N (0.024 mol), solubilized in 20 mL of CH2Cl2at a temperature of 0 °C, maintained by an ice bath,2-chloroacetyl chloride (0.024 mol) was slowly added. Theice bath was then removed, and the reaction stayed underagitation for 6 h at room temperature. The reaction mixturewas monitored by TLC (hexane/methyl acetate 1:1). At theend of reaction, the reaction mixture was concentrated atreduced pressure and cold water was added to the reactionmixture, causing the formation of a solid. The solid wasthen filtered and washed with cold water (3 × 20 mL), andthe final product was purified by recrystallization with anethanol/water (1:1) mixture. α-Chloro-N-phenylacetamide (2a)Yield: 93%,
References:
Souza, Helivaldo D.S.;De Sousa, Roxana P.F.;Lira, Bruno F.;Vilela, Raquel F.;Borges, Nathalie H.P.B.;De Siqueira‑Junior, José P.;Lima, Edeltrudes O.;Jardim, Jeane U.G.;Da Silva, Gracielle A.T.;Barbosa‑Filho, José M.;De Athayde‑Filho, Petrônio F. [Journal of the Brazilian Chemical Society,2019,vol. 30,# 1,p. 188 - 197]