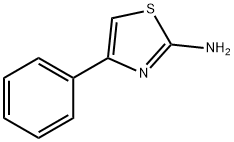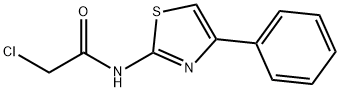
2-Chloro-N-(4-phenyl-thiazol-2-yl)-acetamide synthesis
- Product Name:2-Chloro-N-(4-phenyl-thiazol-2-yl)-acetamide
- CAS Number:5039-16-7
- Molecular formula:C11H9ClN2OS
- Molecular Weight:252.72
Yield:5039-16-7 95%
Reaction Conditions:
Stage #1: chloroacetyl chloridewith triethylamine in tetrahydrofuran at 0; for 0.25 h;Inert atmosphere;
Stage #2: 2-Amino-4-phenylthiazole in tetrahydrofuran at 0 - 20; for 13 h;
Steps:
General procedure for the synthesis of N-(4-aryl-1,3-thiazol-2-yl)-2-chloro-acetamide 4a-e.
General procedure: A solution of triethylamine (7.5 mmol in 5 mL of THF) was added to a twin-necked round-bottom flask under argon atmosphere, cooled to 0 °C, in a 2-chloroacetyl chloride solution (3 mmol in 3 mL of THF). The mixture was stirred for 15 min, followed by the addition of 2-amino-4-aryl-1,3-thiazole (1.5 mmol in 1 mL of THF). The reaction was kept under stirring for 1 h at 0 °C and for additionally 12 h at room temperature. Following the 12 h, the reaction content was diluted in dichloromethane (50 mL) and washed with aqueous 1M NaOH (2 x 10mL) and saturated aqueous NaCl (10 mL). The organic layer was dried over anhydrous MgSO4 and filtered. Finally, the solvent was removed under reduced pressure. When necessary, the crude product was purified by flash chromatography on silica gel, employing a mixture of hexane/ethyl acetate (70:30) as the eluent.
References:
Wolf, Lucas;Quoos, Natália;Mayer, Jo?o C.P.;De Souza, Diego;Sauer, André C.;Meichtry, Luana;Bortolotto, Vandreza;Prigol, Marina;Rodrigues, Oscar E.D.;Dornelles, Luciano [Tetrahedron Letters,2016,vol. 57,# 9,p. 1031 - 1034] Location in patent:supporting information
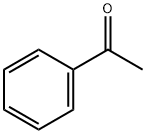
98-86-2
743 suppliers
$9.00/5g

5039-16-7
14 suppliers
inquiry

70-11-1
288 suppliers
$10.00/25g

5039-16-7
14 suppliers
inquiry