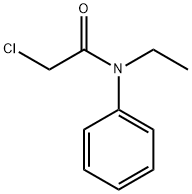
2-chloro-N-ethylacetanilide synthesis
- Product Name:2-chloro-N-ethylacetanilide
- CAS Number:39086-61-8
- Molecular formula:C10H12ClNO
- Molecular Weight:197.66
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 0.333333 h;Inert atmosphere;
Steps:
Ethyl (4-Methoxyphenyl)glycylglycinate (1f)
To a suspension of ethylglycinate hydrochloride (3.58 g,25.6 mmol) in CH2Cl2 (50 mL) were added Et3N (10.7 mL,76.9 mmol) and chloroacetyl chloride (3.05 mL, 38.4 mmol) at0°C and stirred at room temperature for 20 min. After that,sat. NaHCO3aq was added at 0°C and the reaction mixture wasextracted with CH2Cl2, dried over Na2SO4 and evaporated invacuo to afford the chloroacetamide intermediate as a darkblack solid. This intermediate was directly used for the nextstep without further purifications. To a solution of chloroacetamide in CH3CN (50 mL)were added p-anisidine (4.90 g, 39.8 mmol), K2CO3 (4.04 g,29.2 mmol) and KI (4.25 g, 25.6 mmol) at room temperatureand stirred at the same temperature for 24 h. After that, thereaction mixture was evaporated in vacuo, extracted withEtOAc, dried over Na2SO4, evaporated in vacuo and purifiedby silica gel column chromatography (hexane-EtOAc=1 : 1 to1 : 2) to afford 1f (3.21 g, 47% in two steps) as a pale brown powder.
References:
Inokuma, Tsubasa;Jichu, Takahisa;Nishida, Kodai;Shigenaga, Akira;Otaka, Akira [Chemical and Pharmaceutical Bulletin,2017,vol. 65,# 6,p. 573 - 581]
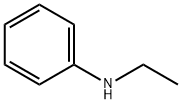
103-69-5
248 suppliers
$13.00/100g

79-04-9
352 suppliers
$12.00/5g

39086-61-8
16 suppliers
$28.60/25mg

103-69-5
248 suppliers
$13.00/100g
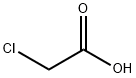
79-11-8
10 suppliers
$27.00/25g

39086-61-8
16 suppliers
$28.60/25mg
