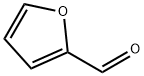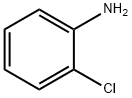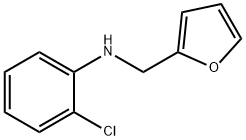
2-chloro-N-(furan-2-ylmethyl)aniline synthesis
- Product Name:2-chloro-N-(furan-2-ylmethyl)aniline
- CAS Number:849032-51-5
- Molecular formula:C11H10ClNO
- Molecular Weight:207.66
Yield:849032-51-5 86 %Chromat.
Reaction Conditions:
with hydrogen in tetrahydrofuran at 120; under 4500.45 Torr; for 6 h;Autoclave;
Steps:
2.4 Hydrogenation Process
In a typical run for selective hydrogenation reduction offurfural to furfuryl alcohol, 0.50mmol furfural, 50.0mgcatalyst, and 5.0mL THF were put into a 25mL stainlesssteel autoclave. After three purging of the reactor with pureH2,the reactor was then pressurized with H2and heatedto 100°C for 6.0h with stirring at 600rpm. Likewise, theone-pot reductive amination of furfural was carried out in a25mL stainless steel autoclave, followed by the introductionof 0.50mmol furfural, 1.0mmol aniline, 50.0mg catalyst,and 5.0mL THF. The reactor was further purged with H2three times and treated at 110°C with stirring of 600rpm.After the reaction, the autoclave reactor was cooled downto 30°C. The used catalyst was firstly separated from thereaction mixture through filtration. The recycled catalystwas washed with ethanol five times and reused after drying.The liquid products were qualitatively identified throughgas chromatography-mass spectrometry (GC-MS, ThermoTrace 1300 GC-ISQ) and quantitatively analyzed usinginternal standard dodecane by gas chromatography (ThermoTrace 1310) with flame ionization detector.
References:
Chen, Wen-Ting;Gao, Zi-Teng;Guan, Hua;Han, Song;Li, Zhang-Min;Tao, Duan-Jian [Catalysis Letters,2022]