
2-(CHLOROMETHYL)-6-METHYL-1,3-BENZOXAZOLE synthesis
- Product Name:2-(CHLOROMETHYL)-6-METHYL-1,3-BENZOXAZOLE
- CAS Number:143708-33-2
- Molecular formula:C9H8ClNO
- Molecular Weight:181.62

2835-98-5
242 suppliers
$8.00/5g

79-04-9
352 suppliers
$12.00/5g

143708-33-2
31 suppliers
$45.00/50mg
Yield:143708-33-2 88%
Reaction Conditions:
Stage #1: 2-Hydroxy-4-methylanilin;chloroacetyl chloride in 5,5-dimethyl-1,3-cyclohexadiene at 0; for 0.5 h;
Stage #2: with triethylamine in 5,5-dimethyl-1,3-cyclohexadiene; for 3 h;Reflux;
Steps:
6.1 Step 1:Preparation of 2-(chloromethyl)-6-methylbenzo[d]oxazole
500 mg of 6-amino-m-cresol (4.00 mmol) was dissolved in 15 ml of xylene, and 0.48 ml of chloroacetyl chloride (6.09 mmol) was added at 0 °C, followed by stirring.
After 30 minutes, 0.62 ml of triethylamine (4.47 mmol) was added, and the mixture was stirred under reflux for 3 hours.
After completion of the reaction, the resultant was extracted with 100 ml of ethyl acetate, washed with 100 ml of an inorganic salt solution, dried over anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure.
The residue was purified by column chromatography (hexane:ethyl acetate = 20:1, v/v) to give 640 mg of the title compound (3.52 mmol) in 88% yield.
Rf = 0.64 (hexane: ethyl acetate = 9:1, v/v)
1H NMR (500 MHz, CDCl3) δ 7.52 (dd, J = 8.9, 4.8 Hz, 1H), 7.45 (dd, J = 8.1, 2.5 Hz, 1H), 7.16 (ddd, J = 9.1, 8.9, 2.5 Hz, 1H), 4.76 (s, 2H)
References:
EP3892623,2021,A1 Location in patent:Paragraph 0124-0125

2835-98-5
242 suppliers
$8.00/5g
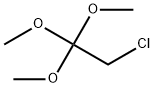
74974-54-2
173 suppliers
$6.00/1g

143708-33-2
31 suppliers
$45.00/50mg